- Home
- Equipment
- north america
- cell transplant
Refine by
Cell Transplant Equipment Supplied In North America
208 equipment items found
Manufactured by:Trailhead Biosystems Inc. based inBeachwood, OHIO (USA)
We are currently developing a novel protocol that will use only small molecules to make human medium spiny neurons from human (non-embryo) induced pluripotent stem cells (iPSCs). We will use our medium spiny neurons as the foundation for a cell transplantation therapy for treating Huntington’s Disease, which we are ...
Manufactured by:IN8Bio Inc. based inNew York, NEW YORK (USA)
INB-100, our DeltEx Allo, is an allogeneic product candidate, initially developed for the treatment of patients with acute leukemia undergoing hematopoietic stem cell transplantation (HSCT). This is the first clinical trial of an expanded and activated allogeneic gamma delta T cell ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-155 is an investigational, oral, rationally designed, fermented microbiome therapeutic designed to reduce the incidence of gastrointestinal infections, bacteremia, and graft versus host disease (GvHD) in immunocompromised patients, including patients receiving allogeneic hematopoietic stem cell transplantation ...
Manufactured by:Pluristem Therapeutics Inc. based inHaifa, ISRAEL
PLX-R18 cells release a combination of therapeutic proteins in response to a damaged or poorly functioning hematopoietic system; this system creates the blood cells that protect us from infection, uncontrolled bleeding and anemia. PLX-R18 is currently in a Phase I clinical trial to treat incomplete hematopoietic recovery following Hematopoietic ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Narsoplimab (OMS 721) is a high-affinity fully human immunoglobulin gamma 4 (IgG4) monoclonal antibody that binds MASP-2 and blocks lectin pathway activation. Narsoplimab can be used in research of hematopoietic stem-cell transplantation and ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
These mutations cause the misfolding and dysfunction of IDUA, which leads to the toxic buildup of large sugars in the bone, cartilage, cornea, heart and central nervous system (CNS). There is currently no cure for these patients; however, enzyme replacement therapy and hematopoietic stem cell transplant are used to manage the non-CNS symptoms. Gain is developing ...
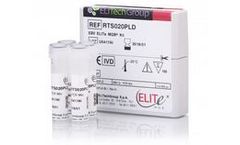
Manufactured by:ELITechGroup based inPuteaux, FRANCE
Epstein-Barr Virus (EBV) is a widespread herpesvirus, generally asymptomatic or sometimes responsible for mild infection like mononucleosis. After solid organ or hematopoietic stem cell transplant, EBV reactivation is a common cause of malignant transformation, called Post-Transplant Lymphoproliferative Disorder “PTLD”. EBV viral load ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Very limited and investigational symptomatic treatment options include substrate reduction therapy, enzyme replacement therapy, bone marrow transplantation, stem cell transplantation and gene therapy to address non-CNS symptoms. Gain is developing allosteric regulators that are designed to decrease toxic substrate accumulation in organs and ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Very limited and investigational symptomatic treatment options include substrate reduction therapy, enzyme replacement therapy, bone marrow transplantation, stem cell transplantation and gene therapy to address non-CNS symptoms. Gain is developing allosteric regulators that are designed to decrease toxic substrate accumulation in organs and ...
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Perform consistent and compliant biotherapeutics characterization faster than ever. With the PA 800 Plus system, you can confidently safeguard the success of your biologics. Run multiple characterizations from a single system that biopharma labs depend on, and produce qualitative and quantitative analyses, with speed and ...
Manufactured by:Xcell Biosciences Inc. based inSan Francisco, CALIFORNIA (USA)
Cell therapy manufacturing re-imagined. An automated, closed system for the production of autologous cell therapies. The KALI Cell Foundry is currently available for licensing ...
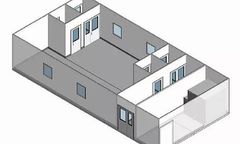
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
G-CON has developed a portfolio of six standardPOD Cleanrooms. These PODs are fully designed, allowing them to be mass-produced. Delivery time is as little as 3 months with the future goal of being able to maintain an inventory of ready to deliver PODs. Mass production of standardPODs will also lower costs. We believe cleanroom infrastructures have to become an off-the-shelf piece of ...
Manufactured by:Obsidian Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
Engineered with membrane-bound IL15, Eliminates toxic IL2 regimen, increasing patient accessibility to TIL therapy, Drives improved persistence, IL15 expression controlled by acetazolamide (ACZ), via cytoDRiVE® ...
Manufactured by:Ardigen based inKraków, POLAND
Accelerate discovery and improve safety of TCR therapies using Artificial Intelligence. Following the unique opportunity for curing patients provided by the development of cell therapies (e.g. TCR discovery), Ardigen has set on the path to advance the field with its Artificial Intelligence platform. Many challenges stand in the way of successful therapy discovery and development. Let us know how ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Sacituzumab govitecan-hziy 180 mg for injection. ...
Manufactured by:Orgenesis Inc. based inGermantown, MARYLAND (USA)
We leverage closed, automated processes that are validated for reliability and ...
Manufactured by:Polysciences, Inc. based inWarrington, PENNSYLVANIA (USA)
MAXgene GMP Transfection Reagent is a cGMP transfection reagent for the development and manufacturing of viral vectors for cell-and gene-based therapies. It is an ideal reagent in HEK293 and CHO systems for the manufacture of AAVs, LVs and recombinant proteins. MAXgene GMP capitalizes on the efficiency and scalability of Polysciences’ PEI MAX while adding the validation process and ...
Manufactured by:Charter Medical, LLC based inWinston-Salem, NORTH CAROLINA (USA)
Charter Medical’s Advect® Fluid Transfer Sets are designed to support the quality and manufacturing requirements of personalized care. The transfer sets are intended for sterile transfer of bone marrow mononuclear cells from one container to another. ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
The Celution System is a medical technology developed by Cytori to automate and standardize the extraction and concentration of adult Adipose-Derived Regenerative Cells (ADRCs) in a clinical setting. The Celution System enables real-time access to autologous, clinical-grade ADRCs at the point-of-care facilitating cell therapy through the reimplantation or reinfusion of a patient’s own ...
Manufactured by:Altucell, Inc. based inNew York, NEW YORK (USA)
Encapsulated cell therapy is an emerging area of biopharmaceutical research that aims to unleash the therapeutic potential of cells to treat serious diseases without the need for immunosuppression. Altucell has developed patented, proprietary engineered cells that are combined with a patented encapsulation technology to create effective ...