- Home
- Equipment
- north america
- clinical screening trial
Refine by
Applications
- Medical cannabis solutions for regulatory pathway sector
- Vaccine for COVID-19
- Medical cannabis solutions for mental health costs sector
- Freeze-Dried Hemostatic for Patients
- Microbiota for Restoration Therapy
- Microbiota Based Product for Physician-Sponsored Studies
- Artificialiris Medical Device for Patient
- Solution for Clinical Trials
- Solution for Access to Investigational Medicines
- Exhaled Breath and Gas Monitoring Instruments for Smoking Cessation industry
- Microbiome Therapeutics for Patients and Physicians
- Flagship Coil for Major Depressive Disorder (MDD) Treatment
- Flagship Coil for Anxious Depression Treatment
- Flagship Coil for Obsessive-Compulsive Disorder (OCD) Treatment
- Flagship Coil for Smoking Addiction Treatment
- Flagship Coil for Bipolar Disorder Treatment
- Flagship Coil for Post Traumatic Stress Disorder Treatment Treatment
- Flagship Coil for Schizophrenia (Negative Symptoms) Treatment
- Flagship Coil for Alzheimer’s Disease Treatment
- Flagship Coil for Autism Disorder Treatment Technology
- Potent, Long-Lasting, Infusion Therapy for Patients
- Colovac - Flexible Endoluminal Bypass Sheath for Patients
Clinical Screening Trial Equipment Supplied In North America
419 equipment items found
Manufactured by:Aldevron, LLC based inFargo, NORTH DAKOTA (USA)
Find the Right Backbone for your Application. Aldevron, your trusted partner since 1998, has several cloning backbones available. These cloning backbones are empty vectors that can be modified to fit your needs. Each vector is available at research grade and is royalty and license free from research through commercial applications. pALD-CV42 and pALD-CV77 are ideal for researchers who do not have ...
Distributed by:Optimum Therapeutic Solutions based inIrving, TEXAS (USA)
Co Enzyme Q10 offers 100mg of highly bioavailable ubiquinone coenzyme (CoQ10) in easy-to-swallow softgels. It is manufactured via a new, proprietary emulsification process that uses all-natural ingredients, including vitamin E, medium chain triglycerides (MCT) and lecithin that is free of polysorbate 80. Superior bioavailability has been demonstrated in an in-house human clinical trial, showing ...
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
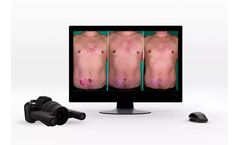
The 3D LifeViz® Series offers a range of high-quality 3D stereoscopic systems to consistently document any part of the body with unmatched accuracy and precision. All of our 3D systems are fully handheld and rely on a built-in ranging light system to ensure high-quality, reproducible images. Combined with our image analysis services, the 3D LifeViz® systems offer a powerful solution to ...
by:Forest Devices based inPittsburgh, PENNSYLVANIA (USA)
Forest Devices has developed a disruptive technology: the first objective, electronically-determined test for stroke for use by EMS. shown an increase in diagnostic accuracy of 92% in pilot clinical trials of nearly 300 people expected to reduce — and possibly eliminate in some cases — disabilities, rehabilitation costs and even death from stroke expected to reduce or avoid hospital ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
Patients with MoCD Type A have mutations in the MOCS1 gene leading to deficient MOCS1A/B dependent synthesis of the intermediate substrate, cPMP. Substrate replacement therapy with fosdenopterin provides an exogenous source of cPMP, which is converted to molybdopterin. Molybdopterin is then converted to molybdenum cofactor, which is needed for the activation of molybdenum-dependent enzymes, ...
by:Inteliquet, An IQVIA Business based inDover, DELAWARE (USA)
OncWeb is proven technology used by community and academic research centers, IDNs and hospitals to improve feasibility and patient-matching operations. OncWeb reduces the manual burden of selecting the most suitable clinical trials with a user-friendly feasibility tool. Through the power of natural language processing and proprietary data parsers, OncWeb harnesses unstructured clinical data ...
Manufactured by:Aldevron, LLC based inFargo, NORTH DAKOTA (USA)
Aldevron provides pALD Lenti packaging plasmids through our partnership with OXGENE, who have optimized them for lentiviral vector production. They are immediately available at all quality levels, eliminating the need to manufacture custom batches. This reduces your costs and significantly reduces time to manufacture vectors for clinical ...
Manufactured by:Entero Therapeutics, Inc. based inBoca Raton, FLORIDA (USA)
Latiglutenase (IMGX003) is an orally administered mixture of two gluten-specific recombinant proteases that degrades gluten proteins into small physiologically irrelevant fragments, and is to be administered as an adjunct to a gluten-free diet (GFD). As illustrated in the below figure, one protease (IMGX001) is effective at breaking glutamine-leucine bonds and the other protease (IMGX002) targets ...
by:saNOtize Research and Development Corp. based inVancouver, CANADA
VirX and enovid are identical formulations based on our NORS (Nitric Oxide Releasing Solution) platform technology. They have the same active ingredients and mechanisms of action as those nasal therapeutics used in our clinical ...
by:saNOtize Research and Development Corp. based inVancouver, CANADA
VirX and enovid are identical formulations based on our NORS (Nitric Oxide Releasing Solution) platform technology. They have the same active ingredients and mechanisms of action as those nasal therapeutics used in our clinical ...
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
The 2D DermaViz® is a fully handheld photography device equipped with fixed optics and a built-in ranging light system, ensuring high-quality, reproducible images. Designed for clinical staff use, this system is easy-to-use and delivers consistent results regardless of the extent of previous experience in photography. ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
CX-188 is our wholly owned PD-1-targeting Probody therapeutic. As with anti-PD-L1 therapies, PD-1 monotherapy and combinations have been associated with significant toxicities. Our preclinical studies have shown that CX-188 has the potential for an improved therapeutic index relative to PD-1 antibodies. The IND for CX-188 was cleared by the FDA. Following a program and portfolio prioritization, ...
by:Intelerad based inRaleigh, NORTH CAROLINA (USA)
A comprehensive image management suite designed for the modern researcher, featuring adaptable intake workflows, enhanced accessibility, and specialized tools to manage imaging research data. ...
by:Curavit Clinical Research based inBoston, MASSACHUSETTS (USA)
The Curavit DCT Platform is the digital manifestation of our decentralized clinical trial services. From operations, through data collection, to patient experience, our proprietary platform manages every aspect of your trial and provides insight into your data. Our platform, reflects our expert processes, is built from industry-leading technology, configured for each trial, deployed to each ...
Manufactured by:Definitive Healthcare, LLC. based inFramingham, MASSACHUSETTS (USA)
Get intelligence on more than 124,000 physician groups to quickly understand an entire organization and improve your marketing and sales efforts. Leverage up-to-date organizational profiles with data curated from more than 10 different public, private and proprietary sources. Identify the most strongly affiliated physician members, relationships with other organizations by firm type, key ...
Manufactured by:Lenstec, Inc. based inSt Petersburg, FLORIDA (USA)
This lens is currently in a US FDA-approved clinical trial. The Lenstec SBL-3 Segmented Bifocal Lens is a presbyopic lens that is intended to provide superior HD vision. It is a asymmetric segmented multifocal IOL (MIOL) that is designed to improve contrast sensitivity, minimize halos and glare, and provide superior near, intermediate and distance vision compared to other presbyopic ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
The first and only FDA-approved prescription medication for the treatment of primary hyperoxaluria type 1 (PH1). OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine in children and ...
by:Evofem Biosciences, Inc. based inSan Diego, CALIFORNIA (USA)
We are collaborating with Orion Biotechnology to evaluate the compatibility and stability of Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product candidate for indications including the prevention of human immunodeficiency virus (HIV) in women. This collaboration will focus on determining compatibility and stability ...
Manufactured by:IN8Bio Inc. based inNew York, NEW YORK (USA)
INB-400, our DeltEx Allo DRI, is a preclinical product candidate expanding the application of DRI gamma-delta T cells into other solid tumor types through the development of allogeneic or “off-the-shelf” DeltEx DRI therapy. We aim to take the clinical results from the ongoing INB-200 and INB-100 clinical trials to provide the safety data to support an IND submission for INB-400. The ...
Manufactured by:Perspectum based inOxford, UNITED KINGDOM
Our digital pathology offering uses computer-generated, quantitative metrics to standardise biopsy analysis. We partner with ProPath to combine best-in class imaging with best-in-class pathology into a single integrated workflow for more objective, quantitative biopsy data. Our digital pathology service streamlines and improves decision-making in clinical trials with expert central review and ...