- Home
- Equipment
- north america
- kidney transplant
Refine by
Kidney Transplant Equipment Supplied In North America
18 equipment items found
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to ...
by:CareDx, Inc based inSouth San Francisco, CALIFORNIA (USA)
AlloSeq cfDNA is a minimally invasive blood test that measures the amount of donor-derived cell-free DNA (dd-cfDNA) for transplant surveillance. It is currently only available outside of the United ...
Manufactured by:cellvie Inc. based inHouston, TEXAS (USA)
Mitochondria dysfunction has been tied to a host of medical conditions, including heart disease, stroke, diabetes, Parkinson's and Alzheimer's. But mitochondria have, for the most part, eluded treatment. This compelled Dr. McCully and his team to think in new ...
Manufactured by:Medcomp based inHarleysville, PENNSYLVANIA (USA)
The catheter can act as a bridge device until permanent access, if applicable, is matured and ready for use, or until kidney transplantation. allows vascular access in patients requiring hemodialysis or apheresis for three weeks or longer who do not have functional permanent vascular access or are not candidates for permanent vascular access. The catheter can act ...
Manufactured by:Medcomp based inHarleysville, PENNSYLVANIA (USA)
The catheter can act as a bridge device until permanent access, if applicable, is matured and ready for use, or until kidney ...
Manufactured by:Medcomp based inHarleysville, PENNSYLVANIA (USA)
The catheter can act as a bridge device until permanent access, if applicable, is matured and ready for use, or until kidney ...
Manufactured by:Medcomp based inHarleysville, PENNSYLVANIA (USA)
The catheter can act as a bridge device until permanent access, if applicable, is matured and ready for use, or until kidney ...
Manufactured by:CSL Behring based inKing of Prussia, PENNSYLVANIA (USA)
CMV Immunoglobulin-VF is a sterile, preservative free solution containing 50-70 mg/mL human plasma protein of which at least 98% is immunoglobulin (mainly IgG), with a cytomegalovirus (CMV) immunoglobulin activity of 1.5 million units per vial. The content of CMV immunoglobulin is defined in terms of EIA (enzyme immunoassay) units. One unit is equivalent to the specified volume of a standard CMV ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-1500 is a humanized monoclonal antibody (mAb) directed against CD40-ligand, which is also known as CD154, T-BAM, 5c8 antigen, TRAP and gp39. TNX-1500 incorporates the antigen binding fragment (Fab) region of hu5c8 (recombinant monoclonal antibody to CD40-ligand), that has been extensively characterized at the molecular level in complex with the CD40-ligand. TNX-1500 is being studied and ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Miromatrix is developing bioengineered organs for transplantation, and our technology can be applied across the spectrum of donor needs. We are currently focused on bioengineering transplantable kidneys (MiroKidney) and livers (MiroLiver), but we believe our technology can be used to engineer other in-demand organs, such as lungs, pancreases, ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
Artegraft is a bovine carotid artery graft. Its biological fibrous matrix is processed to enhance long-term patency and provide a tightly woven, cross-linked conduit that is flexible and compliant. For Functional Hemodialysis Access & Lower Extremity ...
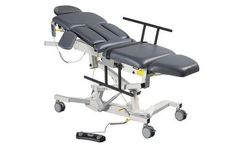
Manufactured by:Biodex Medical Systems, Inc. based inShirley, NEW YORK (USA)
The Econo Ultrasound Table is designed on the same platform as the Ultra Pro with similar features and functions. To make it a more cost-effective alternative it is offered without Trendelenburg positioning or central floor-locking system. The same ergonomics have been incorporated into the design of the Econo Ultrasound Table, making it easy for a sonographer to achieve quality images without ...
Manufactured by:Biodex Medical Systems, Inc. based inShirley, NEW YORK (USA)
The Ultra Pro™ Ultrasound Table is the professional’s choice when performing general ultrasound procedures, including OB/GYN. The table's ergonomics are designed to reduce sonographer fatigue and require less patient repositioning, making it easy to achieve quality images in less time. Fowler positioning which provides the utmost patient comfort, an enhanced central floor-locking ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Mizoribine (NSC 289637), an imidazole nucleoside, inhibits HCV RNA replication with IC50 of approximately 100 μM for anti-HCV activity. Immunosuppressant[1]. Mizoribine, an IMPDH inhibitor, inhibits replication of SARS-CoV with IC50s of 3.5 μg/mL and 16 μg/mL for SARS-CoV Frankfurt-1 and SARS-CoV HKU39849, ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-300-IV is being developed for the treatment of diabetic complications, including diabetic kidney disease known as diabetic ...
Manufactured by:Nikkiso Co., Ltd. based inShibuya-ku, JAPAN
As a pioneer of dialysis machines, Nikkiso has been moving forward with the development of Hemodialysis . In order to propose a comfortable therapeutic environment for everyone who requires Hemodialysis, operations from development, manufacturing and sales, to maintenance are performed consistently at the Nikkiso Group. We promise to continue providing new proposals to customers, while making ...
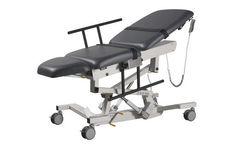
Manufactured by:Biodex Medical Systems, Inc. based inShirley, NEW YORK (USA)
The Sound Pro™ Combination Table brings together the imaging features of both the Ultra Pro and Echo Pro tables. Fully equipped for OB/GYN, general ultrasound and echocardiography procedures, the extra wide tabletop is designed with advanced ergonomics to provide maximum comfort for both patient and sonographer. Designed to conform to natural body extension and sitting position, the ...
Manufactured by:Jubilant DraxImage Inc. dba Jubilant Radiopharma based inKirkland, Montréal, QUEBEC (CANADA)
Each kit consists of reaction vials which contain the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Pentetate Injection for diagnostic use by intravenous injection or for inhalation after the nebulization to radio-aerosol. Available in kits containing 10 reaction ...