- Home
- Equipment
- north america
- medical device sterilization
Show results for
Refine by
Medical Device Sterilization Equipment Supplied In North America
11 equipment items found
Manufactured by:Nordion (Canada) Inc. based inOttawa, ONTARIO (CANADA)
The Parallel Row Pallet Irradiator provides the ideal solution for processing intact pallets of product, reducing material handling labour cost while maximizing throughput. Ideal for both phytosanitary applications and medical device sterilization, the Pallet Irradiator accommodates a variety of product densities and dose uniformity requirements. ...
Manufactured by:Advanced Sterilization Products (ASP) based inIrvine, CALIFORNIA (USA)
Easy-to-read process indicators to differentiate processed from unprocessed medical device packages to be sterilized in STERRAD® Sterilization Systems. SEALSURE® CI Tape and STERRAD™ CI Strips change from red to color (or lighter) indicated by comparator bar when exposed to hydrogen peroxide to differentiate between ...
Manufactured by:Precision Medical Products, Inc. based inDenver, PENNSYLVANIA (USA)
Before the 1960s, smallpox vaccination campaigns relied on unreliable jet injectors that slowed delivery of the vaccine. Precision Medical Products, Inc., changed that with the introduction of the bifurcated needle, which holds a dose of reconstituted smallpox vaccine in its prongs and punctures a person’s skin easily to the ideal depth for delivery. Our engineers designed the needle, and ...
Manufactured by:Metrex Research, LLC. based inOrange, CALIFORNIA (USA)
Pre-cleaner used on medical devices prior to terminal sterilization or high-level disifection. Contains two protease enzymes that offer a broad cleaning action on a variety of protein soils. Inclusion of surfactants provides additional cleaning on carbohydrates, lipid and protein soils. ...
Manufactured by:Galbino Technology Inc. based inMarkham, ONTARIO (CANADA)
The Galbino ZW2211A series filter container has been specifically engineered for optimal performance in hospital sterile supply environments. It is constructed from high-quality materials that facilitate efficient heat conduction and exhibit excellent corrosion resistance while maintaining a lightweight profile. The series offers the convenience of either disposable or reusable filters to enhance ...
Manufactured by:Case Medical, Inc. based inSouth Hackensack, NEW YORK (USA)
The SteriTite Universal Sterilization Container System from Case Medical is a robust solution for reusable medical packaging, designed specifically to enhance efficiency and sustainability in healthcare sterilization processes. As a universal system, these containers are compatible with all sterilization ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Ahlstrom’s effective bacterial-barrier paper for packaging sterilized medical devices is sealable, peelable and ...
Manufactured by:Case Medical, Inc. based inSouth Hackensack, NEW YORK (USA)
The SteriTite Rigid Sterilization Containers represent an advanced and universal solution for medical sterilization needs. These containers are compatible with all sterilization modalities, featuring the latest advancements like digital visualization via 2D barcode scanning. Constructed from durable materials such as anodized aluminum and platinum-cured silicone, these containers offer superior ...
Manufactured by:FCI based inPembroke, MASSACHUSETTS (USA)
The EZYPOR orbital implant is an advanced ophthalmic device crafted from ultra high molecular weight polyethylene (UHMWPE). It is particularly designed for enucleation, evisceration, and secondary implantation procedures. The implant features a porosity range between 40% and 60%, facilitating the optimal colonization of fibrovascular tissues which is crucial for integration and ...
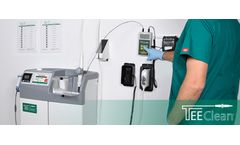
Manufactured by:CS Medical LLC based inCreedmoor, NORTH CAROLINA (USA)
TEEClean Automated TEE Probe Cleaner Disinfector is the first product of its kind to be cleared by the US FDA. Designed exclusively for transesophageal echocardiogram ultrasound probes (TEE), TEEClean provides both manufacturers recommended cleaning and high-level disinfection of soiled TEE probes in the same device. Current methods require manual cleaning to be completed so as to achieve proper ...
Manufactured by:Hensler Surgical Products based inWilmington, NORTH CAROLINA (USA)
ConCelltrate 100 is an osteoinductive bone matrix. ConCelltrate 100 products are verified for osteoinductivity post-sterilization prior to release for distribution. In-vivo test results demonstrate all 5 bone forming elements present (chondrocytes, osteocytes, bone marrow cells, cartilage, and new bone) in our implants. ConCelltrate® 100 does not contain any extrinsic carriers and is made ...