- Home
- Equipment
- north america
- medical devices
Refine by
Applications
- Clean air solutions for allergens area
- Clean air solutions for pollution area
- Clean air solutions for bad odor area
- Clean air solutions for bacteria area
- Clean air solutions for virus area
- Solutions for Pharmaceutical
- Microbial Reduction Solutions for Cosmetic and Packaging
- Flagship products for therapeutic industry
- Diagnostic Solutions for Depression
- Artificialiris Medical Device for Patient
- Medical solutions for MiniTC and mini-stem system sector
- Home Sleep Testing for Medical Professionals
- Home Sleep Testing for Sleep Apnea
- IONICON PTR-TOF instruments deployed for COVID-19 detection in breath
- Medical Device Solutions for Full Lifecycle
- Medical Devices for Moderate to Severe Heart Failure Treatment
- Medical Devices for Severe or Advanced Stage Heart Failure Treatment
- Medical Devices for Causes Heart Failure
- Medical Devices for Heart Failure Symptoms
- Medical Devices for Diagnosis and Evaluation
- Acute Kidney Injury (AKI) Device for Reduction of Galectin-3 Levels
- Acute Kidney Injury (AKI) Device for Plasmapheresis Therapy
- Acute Kidney Injury (AKI) Device for Patient Selective Apheresis
- Technology for Surface Modification and Drug Delivery
- Medical Device Solutions for Opportunity Realization
- Multi-Function Ventilator for Healthcare Professionals
- Solutions for Glaucoma and Intraocular Pressure (IOP)
- Solution for Ultrafast Laser Spectroscopy / Imaging
- Solution for High-Speed 4D Imaging
- Home Sleep Monitor for Clinical Validation
- Medical Devices for Medical Professionals
- Medical Devices for Patients
- Medical Devices for Chronic Venous Insufficiency: Eliminating Pain
- Medical Devices for Breast Cancer-Related Lymphedema
- Medical Devices for Lipedema
- Ultrasound measurement instrumentation solutions for diagnostic sector
- Ultrasound measurement instrumentation solutions for physiotherapy sector
Medical Devices Equipment Supplied In North America
815 equipment items found
by:Body Scientific International, LLC. based inIngleside, ILLINOIS (USA)
Body Scientific can improve your user manuals, and inservice training with interactive models and applications. Improve the use of your products, provide clear and concise problem solving solutions using AR or VR. ...
by:MC10 based inLexington, MASSACHUSETTS (USA)
BioStamp nPoint is an FDA 510(k) cleared medical device designed to collect medical grade, clinical quality bio-metric, physiological and eCOA data in a clinical trial ...
Manufactured by:3B Medical, Inc. based inSarasota, FLORIDA (USA)
Updated BiLevel design with a smaller lightweight body, the reliability you've come to expect with a compact look and integrated modem ...
Manufactured by:Plasma Surgical, Inc. based inRoswell, GEORGIA (US) (USA)
PlasmaJet delivers effective control to surgeons: Allows enhanced visualization, opening, and dissection of tissue planes. Controlled tissue effect enables use on or around sensitive structures, leading to more complete disease removal. Less than 0.3 mm depth of thermal effect on key structures such as bowel, ovary, and fallopian tubes. Less than 0.5 mm lateral thermal spread. Sealing of small ...
by:Qualityze Inc based inTampa, FLORIDA (USA)
Safety and quality are non-negotiable in the medical devices industry, that’s why we developed ISO 13485. Regulatory requirements are increasingly stringent throughout every step of a product’s life cycle, including service and delivery. Increasingly, organizations in the industry are expected to demonstrate their quality management processes and ...
Manufactured by:Anthogyr Manufacturing based inSallanches, FRANCE
Machining and assembly, and all subsequent phases up to decontamination and packaging. Products are supplied sterile. They are packaged in a clean room for absolute cleanliness and contamination control. Particulate testing is performed and microbial contamination is assessed in a controlled environment to guarantee the quality of our products. To continue to provide exceptional products of the ...
Manufactured by:Bio Molecular Systems based inUpper Coomera, AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
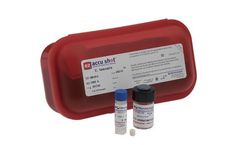
Manufactured by:Microbiologics based inSt. Cloud, MINNESOTA (USA)
Let’s face it; Growth Promotion Testing of culture media is likely not at the top of your "favorite lab activities" list. We get it, which is why we created EZ-Accu Shot. It provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 ...
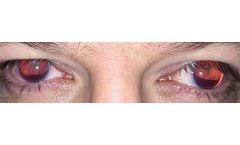
Manufactured by:VEO Ophthalmics, LLC based inCincinnati, OHIO (USA)
Managing congenital aniridia and acquired iris defects — including, but not limited to, traumatic iris defects and traumatic mydriasis — is often challenging. When iris reconstruction is necessary, the CUSTOMFLEX® ARTIFICIALIRIS is a unique device that offers both surgeons and patients important ...
Manufactured by:Engenious Design based inPrairie Village, KANSAS (USA)
Our specialty is medical device development and design. We thrive on innovation, and our team is passionate about designing and creating new products; and improve existing ones! Engenious helps medical device companies apply the latest technology in medical diagnostic and therapeutic devices. Our ...
Manufactured by:Jamak based inWeatherford, TEXAS (USA)
Silicone medical devices. Silicone Rubber Elastomer Industrial Consumer Design Sourcing Logistics ...
Manufactured by:Cenorin, LLC based inKent, WASHINGTON (USA)
Drying is an essential step when reprocessing reusable medical devices for their next safe use. Cenorin drying chambers have supported reprocessing for more than 40 years. Our dryers offer a choice of capacities along with accessories that help enhance the drying of many device types and ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
Gelsoft™ Plus is a surgical solution that enables you to achieve anatomical equilibrium in the ...
Manufactured by:SakoMed, LLC based inLaguna Niguel, CALIFORNIA (USA)
The ZOLL E Series defibrillator medical devices were designed by professionals for professionals. No other full-featured defibrillator was designed specifically for the rigors of your EMS environment. No other defibrillator offers the configurable options of the E Series. ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
Gelweave™ delivers a wide range of solutions for the thoracic ...
Manufactured by:Plasticase, Inc. based inTerrebonne, QUEBEC (CANADA)
NANUK PROTECTS MEDICAL EQUIPMENT AT ALL COSTS. Known as the world’s best protective cases, NANUK safeguards your lifesaving equipment so your users can count on them to work without fail. DO OR DIE PROTECTION:NANUK indestructible cases safeguard fragile, sophisticated equipment from the repeated blows, drops and shocks that happen during emergencies. Crushproof: Made from crushproof ...
Manufactured by:Modulight Corporation based inTampere, FINLAND
ML6710i is a medical laser device for ophthalmology. ML6710i is designed for treatments in various ophthalmic indications. The laser is controlled intuitively from iPad app – the wireless connection allows flexibility for the treatment setup. ...
Manufactured by:Integer Holdings Corporation based inPlano, TENNESSEE (USA)
As a Class III Medical Device manufacturer, our OEM partners trust us to custom design, develop and manufacture their implantable pulse generators (IPG) and lead systems. We honor that trust with a focus on accelerating time to market and reducing program risk and commercialization costs. IPGs are an integral part of our history, in the last 40 of our 70+ years, ...
Manufactured by:Cenorin, LLC based inKent, WASHINGTON (USA)
Cenorin’s line of custom configured dryers efficiently process a variety of medical devices and equipment. The Series 130 is comprised of two models, the 135 (with a solid back) and the 136 (with a back door, or “pass-through”). Both offer a wide variety of options and accessories tailored to meet your specific reprocessing needs. ...
Manufactured by:Cenorin, LLC based inKent, WASHINGTON (USA)
Cenorin’s line of custom configured dryers efficiently process a variety of medical devices and equipment. The Series 1000 is comprised of two models, the 1009 (with a solid back) and the 1010 (with a back door, or “passthrough”). Both offer a wide variety of options and accessories tailored to meet your specific reprocessing ...