- Home
- Equipment
- north america
- open heart
Show results for
Refine by
Open Heart Equipment Supplied In North America
22 equipment items found
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
An innovative, sternal closure system designed to approximate, compress and rigidly fixate the sternum following open-heart surgery in patients with poor bone ...
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
SternaLock Blu is specifically designed by cardiothoracic surgeons for primary sternal closure following median sternotomies for open-heart procedures. Zimmer Biomet has nearly two decades of clinical experience. Explore the legacy studies and whitepapers of our primary closure ...
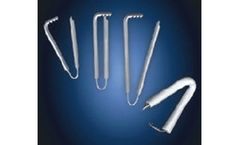
Manufactured by:Remington Medical Inc. based inAlpharetta, GEORGIA (US) (USA)
Disposable screw-down extension cable with Remington Safety Separator for temporary pacing of open hearts. The FL-601-97 Fas-Loc accepts epicardial pacing wires at one end and plugs into a Medtronic 5348 or 5388 EPG or older EPG with an adapter (Medtronic 5101 or Remington ADAP I ...
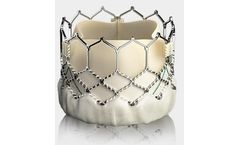
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Jarvik Heart, Inc. based inNew York, NEW YORK (USA)
A total artificial heart is built to replace both ventricles of the living heart. The entire biological heart (except for the atria) is usually removed, and the man-made device is positioned in its place. Most total artificial hearts mimic the natural heart’s rhythm, drawing and pumping blood in pulses. ...
Manufactured by:Newmedical Technology Inc. based inNorthbrook, ILLINOIS (USA)
2" x 8" silicone strips fill the need for a strip bigger than our 1" x 6" strips and shorter than our 2" x 24" abdominal strip. Designed for scars 7" or less, including those resulting from mastectomy, open heart surgery, hysterectomy, knee replacement surgery, and spine ...
Manufactured by:Kapp Surgical Instrument Inc. based inCleveland, OHIO (USA)
May be used prophylactically during any cardiac procedure where there is access to the LA appendage for those patients not yet in a-fib to eliminate future need for anticoagulation should a-fib develop. Ease of use – placed in ideal location at the base of the LA appendage during routine open heart surgery without adding significantly to length of operation ...
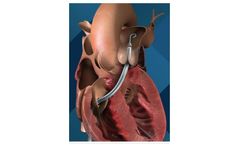
Manufactured by:NaviGate Cardiac Structures, Inc., (NCSI) based inLake Forest, CALIFORNIA (USA)
The AVS is designed to restore valve function in patients presenting with FTR/tricuspid insufficiency without the need for conventional, open-heart ...
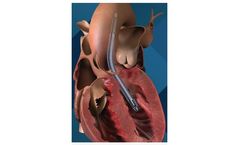
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
The Impella RP heart pump provides temporary, circulatory support for patients who develop right heart failure. It is the only FDA-approved heart pump indicated for patients experiencing acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infraction, heart ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
The Impella 5.5 with SmartAssist heart pump delivers full cardiac support, allowing the heart to rest and enabling the heart to achieve its natural pumping function without additional support. This heart pump is designed for long-duration support, enables patient mobility and optimizes recovery by using real-time ...
Manufactured by:Springboard based inRancho Cordova, CALIFORNIA (USA)
Surgical Device Component Manufacturer; Although Springboard does not make any finished medical devices, we are an industry-leading surgical device component manufacturer. We supply a sizeable number of injection-molded surgical components that go into life-saving surgical devices. At Stack, we understand that our parts are used in devices that can save lives, and that the failure of insert ...
Manufactured by:Puzzle Medical Devices Inc. based inMontreal, QUEBEC (CANADA)
Puzzle Medical is a Canadian company specializing in the development of a minimally invasive long-term hemodynamic transcatheter pump. Puzzle Medical enables mechanical hemodynamic support to increase accessibility, improve patient quality of life and reduce the global economic burden related to the ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ProxiCor is the natural choice for pericardial reconstruction. An extracellular matrix derived from porcine small intestine submucosa (SIS), ProxiCor for Pericardial Closure (PC) regulates the biologic healing immune response to decrease inflammation and stimulate formation of healthy tissue.2,3 Reconstruction of the pericardium using ECM enables surgeons to re-establish essential native anatomic ...
Manufactured by:Quest Medical Inc. based inAllen, TEXAS (USA)
Technology so unique, Quest Medical was awarded eight U.S. patents for this perfusion system. At the heart of the MPS®2 system are Spline-Piston pumps coupled with state-of-the-art pressure sensors enabling unparalleled drug delivery accuracy. The Quest MPS 2 System provides independent control of potassium and carioprotective drugs, providing the flexibility to deliver any cardioplegia ...
Manufactured by:SmartHeart based inHauppauge, NEW YORK (USA)
SmartHeart offers a groundbreaking portable 12-lead ECG device that revolutionizes the ability of healthcare professionals to diagnose and manage heart disease efficiently. The device connects seamlessly to mobile devices via Bluetooth without requiring adhesive gels, using instead just a dab of water. It is battery-powered and lightweight, enhancing portability and making it ...
Manufactured by:Sterlitech Corporation based inAuburn, WASHINGTON (USA)
Sterlitech Polyethersulfone (PES) membrane is a hydrophilic membrane constructed from pure polyethersulfone polymer membrane. PES membrane filters are designed to remove particulates during general filtration. Their low protein and drug binding characteristics also make polyethersulfone (PES) membrane disc filters ideally suited for use in life science ...
Manufactured by:Moray Medical based inMountain View, CALIFORNIA (USA)
Moray Medical’s initial product is the Coral System, which includes a reusable Driver Assembly, a single-use Clipper toolset, and a Trident input device. The Clipper toolset includes a cardiac-valve-therapy edge-to-edge clip implant that is pre-mounted on a single-use fluid-driven steerable delivery catheter. The Driver, a wireless capital-equipment unit about the size of a brick, ...
Manufactured by:Renata Medical based inNewport Beach, CALIFORNIA (USA)
The Minima Stent is designed for use in the treatment of native or acquired pulmonary artery stenoses or coarctation of the aorta. The stent comes packaged on a specifically designed delivery system which allows for sheath-less delivery to the target ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Cardioblate Surgical Ablation Pen is intended to ablate cardiac tissue during cardiac surgery using radiofrequency ...
Manufactured by:emka Technologies S.A.S based inParis, FRANCE
The isolatedHEART system is a versatile solution to investigate small mammalian heart function, in health and disease, or following drug challenges. It provides highly reproducible experimental conditions to ensure survival of the isolated heart during several hours, allowing the simultaneous assessment of cardiac electrophysiological and mechanical parameters (ECG, LVP, perfusion flow & ...