- Home
- Equipment
- north america
- sterility testing
Refine by
Sterility Testing Equipment Supplied In North America
39 equipment items found
Manufactured by:Terragene based inSanta Fe, ARGENTINA
Process Challenge Device (KPCD110) for Ethylene Oxide sterilization ...
Manufactured by:IsoTech Design based inSt-Laurent, QUEBEC (CANADA)
This Primary Engineering Control (PEC) is designed to operate efficiently and safely, adhering to stringent standards such as USP <797/800>, OSHA, and NIOSH. The system integrates advanced aseptic processing and sterility testing methods, utilizing H2O2 for effective decontamination. The ChemoSphere ensures a clean and secure environment, which is crucial ...
Manufactured by:Gloveboxes Group based inAmesbury, MASSACHUSETTS (USA)
Gloveboxes Group specializes in the design and manufacturing of custom isolators tailored for the pharmaceutical industry. These isolators cater to a range of applications, from sterile processes such as weighing, formulation, and sterility testing, to handling toxic substances where operator safety is paramount. The company adheres to stringent ...
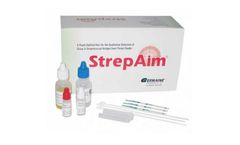
Manufactured by:Germaine Laboratories, Inc. based inSan Antonio, TEXAS (USA)
Ref# 73025 A rapid dipstick test for the qualitative detection of group A streptococcal antigen from throat swabs. Results in 5 minutes Convinient workstation, dipsticks, disposable test tubes, sterile swabs, positive and negative controls. 25 Tests per box, LIA ...
Manufactured by:Jones Medical Instrument Company based inOak Brook, ILLINOIS (USA)
Patented disposable mouthpiece/sensor design minimizes sterilization hassles and maximizes test efficiency. The most cost-effective spirometer mouthpiece/sensor ...
Manufactured by:Jones Medical Instrument Company based inOak Brook, ILLINOIS (USA)
Patented disposable sensor design minimizes sterilization hassles and maximizes test efficiency. The most cost-effective spirometer mouthpiece/sensor ...
Manufactured by:TTI Medical based inSan Ramon, CALIFORNIA (USA)
Accu-Beam® Fixed Focal Endoscope Couplers are a cost effective way to connect any C-mount or SLR camera to an endoscope without compromising on quality. They come in a variety of focal lengths to get the right magnification on any camera and can be sterilized. ...
Manufactured by:Jones Medical Instrument Company based inOak Brook, ILLINOIS (USA)
Patented disposable sensor design minimizes sterilization hassles and maximizes test efficiency. The most cost-effective spirometer mouthpiece/sensor ...
Manufactured by:Sterigenics U.S., LLC based inOak Brook, ILLINOIS (USA)
Electron beam (E-Beam) irradiation is a form of ionizing energy that is characterized by its low penetration and high-dosage rates. The beam – a concentrated, highly charged stream of electrons – is generated by accelerators capable of producing continuous or pulsed beams. As the product/material being sterilized passes the E-Beam, energy from the electrons is absorbed, altering ...
Manufactured by:Sotera Health based inBroadview Heights, OHIO (USA)
Electron beam (E-Beam) irradiation is a form of ionizing energy that is characterized by its low penetration and high-dosage rates. The beam – a concentrated, highly charged stream of electrons – is generated by accelerators capable of producing continuous or pulsed beams. As the product/material being sterilized passes the E-Beam, energy from the electrons is absorbed, altering ...
Manufactured by:Lighthouse Imaging LLC based inWindham, MAINE (USA)
The Lighthouse Imaging Triage Kit adds the EndoScan rigid endoscope visual tester to the EndoLume Measurement Kit. This combination of products is aimed at the Central Sterile Department and provides the capability of performing a complete evaluation of endoscopes in their ...
Manufactured by:Access Biologicals LLC based inVista, CALIFORNIA (USA)
Promotes growth in a variety of mammalian cell lines; Collected from calves >12mo. old and contains double the protein of FBS; .1 micron filtered; sterility, mycoplasma, 9CFR113.53 & virus tested; US ...
Manufactured by:TTI Medical based inSan Ramon, CALIFORNIA (USA)
Available in 13-28mm and 22.5-50mm, Parfocal zoom (with 17.5mm BFD), Focus-control to fine tune your image, Insulated, Sterilization validated, Leak tested, Eyepiece clamp accommodates 32mm eyepieces., Compatible with C-mount or SLR cameras. ...
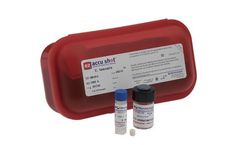
Manufactured by:Microbiologics based inSt. Cloud, MINNESOTA (USA)
Let’s face it; Growth Promotion Testing of culture media is likely not at the top of your "favorite lab activities" list. We get it, which is why we created EZ-Accu Shot. It provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 ...
Manufactured by:Crown Steam Group a Division of the Middleby Corporation based inFuquay Varina, NORTH CAROLINA (USA)
Sterilize glass beakers, pipettes, test tubes and flasks. Metal and plastic instruments, syringes, needles, brushes, rubber gloves, catheters, rubber tubing, dressings, linens, petri dishes and ...
Manufactured by:Crown Steam Group a Division of the Middleby Corporation based inFuquay Varina, NORTH CAROLINA (USA)
Sterilize glass beakers, pipettes, test tubes and flasks. Metal and plastic instruments, syringes, needles, brushes, rubber gloves, catheters, rubber tubing, dressings, linens, petri dishes and ...
Manufactured by:Micropulse, Inc. based inColumbia City, INDIANA (USA)
The Packaging team provides both sterile and non-sterile packaging for orthopedic implants and instruments. Services available include packaging design, testing, and validations. Micropulse offers pre-validated MICROSEAL® options, as well as, custom packaging solutions exclusively for the orthopedic industry. ...
Manufactured by:ZebraSci Inc. based inTemecula, CALIFORNIA (USA)
Residual Seal Force (RSF) is a non-destructive method to evaluate seal tightness in the stopper/seal combination of parenteral vials. Parenteral vial systems consist of a vial, a stopper and an overseal. Capping parameters are a critical aspect during drug product manufacturing. During the sealing process, improper capping can generate cosmetic defects or worse yet, impact Container Closure ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Maintaining the Flow of Progress: Ventricular assist devices (VADs) and circulatory support devices were initially conceived as bridge-to-transplant solutions providing mechanical circulatory support to patients awaiting a donor heart. However, with advances in component technologies, these devices are increasingly being considered as a destination therapy rather than a bridge therapy. As more ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors using the continuous flow Spectra ...