- Home
- Equipment
- north america
- whole blood
Show results for
Refine by
Applications
- Electrolyte Analyzers for Electrolyte Analysis
- Serum Products for Mesenchymal Stem Cells Growth
- Serum Products for Fibroblasts Cell Growth
- Serum Products for Madin-Darby Canine Kidney Cells Growth
- Serum Products for FO Myeloma Cells Growth
- Diagnostic Solutions for Lung Cancer
- Liquid Biopsy Solutions for Breast Cancer
- Diagnostic Solutions for Colorectal Cancer
- Handheld Immunodiagnostic Device for Emergency Room
- Handheld Immunodiagnostic Device for ICU
- Handheld Immunodiagnostic Device for Nursing & Home Care
- Handheld Immunodiagnostic Device for Community & Employee Clinics
- Handheld Immunodiagnostic Device for Rural & Remote Clinics
- Handheld Immunodiagnostic Device for Disaster Relief
- Handheld Immunodiagnostic Device for Defense
- First Rapid Infuser for Sepsis
- First Rapid Infuser for Hypotension / Shock
- First Rapid Infuser for EMS/Military
- First Rapid Infuser for Pediatric
- First Rapid Infuser for Research Grants
Whole Blood Equipment Supplied In North America
306 equipment items found
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics manufactures custom blank controls to fit your manufacturing needs. More Diagnostics is known as the whole blood experts. Quality matrices that enhance your products to provide exceptional ...
Manufactured by:APC Inc/ Lifeline based inAnkeny, IOWA (USA)
AP 300 is a top-quality protein product that is composed of highly digestible and palatable proteins. AP 300 can be used as a partial or complete replacement for other protein ingredients. ...
Manufactured by:LAMPIRE Biological Laboratories, Inc. based inPipersville, PENNSYLVANIA (USA)
Strategically located in close proximity to a wide variety of abattoir facilities, LAMPIRE is able to fill your large volume whole blood needs quickly and efficiently. Our experienced technicians have extensive training on the collection and processing of abattoir whole blood to ensure high-quality ...
Manufactured by:Streck, Inc. based inLa Vista, NEBRASKA (USA)
Sickle-Chex is a positive and negative whole blood control available for use with sickle cell screening tests and hemoglobin electrophoresis. The control does not require reconstitution, which allows it to be used like a patient ...
Manufactured by:Streck, Inc. based inLa Vista, NEBRASKA (USA)
Cal-Chex is a whole blood product manufactured for calibrating multi-parameter hematology analyzers. It is assayed on Beckman Coulter, Abbott, Horiba Medical, Siemens Healthcare Diagnostics and Mindray ...
Manufactured by:Streck, Inc. based inLa Vista, NEBRASKA (USA)
Cell-Free DNA BCT is a direct-draw venous whole blood collection device intended for the collection, stabilization and transport of venous whole blood samples for use in conjunction with cell-free DNA next generation sequencing liquid biopsy assays that have been cleared or approved for use with samples collected in the Cell-Free ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
The CHRONO-LOG® Model 700 Whole Blood/Optical Lumi-Aggregometers are available in a two or four channel configuration. This state-of-the-art Lumi-Aggregometer measures platelet function on patient samples using electrical impedance in whole blood samples or optical density in plasma with the simultaneous measurement ATP ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick Blood Samples can ...
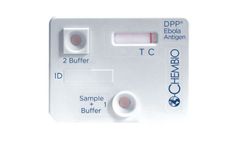
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
A 15 minute rapid test for detection of Ebola Virus Specific Antigen Granted An Emergency Use Authorization (EUA) by the U.S. FDA. For the detection of antigen specific to Ebola Virus in fingerstick whole blood, EDTA venous whole blood and EDTA ...
Manufactured by:Terumo Blood and Cell Technologies, Inc. based inLakewood, COLORADO (USA)
Automation can improve operations and deliver impressive benefits to your blood center. The Reveos system is an easy-to-use platform that automates and integrates the manual steps of whole blood processing: from start to finish, whole blood to platelet concentrate. Plus, you can process four units of ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
A CE-marked single-use immunochromatographic rapid test that detects antibodies to T. cruzi in fingerstick whole blood, venous whole blood, serum, or plasma. ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains 2.5mg of lyophilized adenosine diphosphate. Performs 500 to 1,000 tests in PRP or Whole Blood ...
Manufactured by:SA Scientific, Ltd. based inSan Antonio, TEXAS (USA)
Add 20 ul whole blood to 2 ml of working sickle cell buffer. Read results within 30 ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains 62.5mg of stabilized freeze dried Ristocetin. Performs 62 to 125 tests in PRP or Whole Blood ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains a minimum of 10 units of lyophilized Thrombin from human plasma. Performs 10 to 20 tests in PRP or Whole Blood ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
An FDA Approved, dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, or plasma specimens. ...
Manufactured by:Germaine Laboratories, Inc. based inSan Antonio, TEXAS (USA)
Ref #78200 For the quantitative determination of hemoglobin in non-anticoagulated capillary whole blood or anticoagulated venous whole blood. CLIA ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The FDA-cleared and CE-marked T2Dx® Instrument is a fully automated, walk away, clinical multiplex benchtop diagnostic system capable of running tests directly from whole blood. ...
Manufactured by:Germaine Laboratories, Inc. based inSan Antonio, TEXAS (USA)
Ref #78250 For testing hemoglobin in whole blood, CLIA Waived. Combo Includes: 50 Test Cartridges. 60 Capillary Tubes. 1 Code Chip. For professional use only. For in vitro diagnostic use ...
Manufactured by:410 Medical based inDurham, NORTH CAROLINA (USA)
FAST Deliver 1 unit of whole blood in 2 minutes, PORTABLE Goes from field to hospital, EFFICIENT Easy one-handed operation, SIMPLE Handle and tubing packaged together for quick and easy ...