Show results for
Refine by
Clinical Trials Equipment Supplied In Spain
14 equipment items found
Manufactured by:Suvoda based inConshohocken, PENNSYLVANIA (USA)
Full visibility and automated control. Extend your clinical trial command and control center with Suvoda’s integrated ...
Manufactured by:Suvoda based inConshohocken, PENNSYLVANIA (USA)
Overcome the traditional challenges of electronic outcomes data collection and submission for complex clinical ...
Manufactured by:Suvoda based inConshohocken, PENNSYLVANIA (USA)
Our advanced patient randomization and trial supply management system. Your clinical trial command and control ...
by:STALICLA based inGeneve, SWITZERLAND
STALICLA is advancing a robust pipeline of both proprietary and partnered therapeutic ...
Manufactured by:Biomedal S.L. based inSevilla, SPAIN
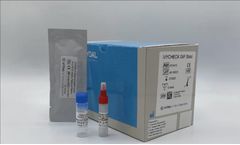
iVYCHECK GIP Stool is an immunochromatographic test designed to detect Gluten Immunogenic Peptides (GIP) in stool samples. Firstly, gluten must be extracted from the sample, and then proceeding to the detection of GIP by reaction with the conjugated antibodies present in the strip. In the case of GIP in the sample due to gluten consumption, a red line will appear (positive result), while the ...
Manufactured by:Histocell based inDerio, SPAIN
ASC pre-differentiated to osteoblasts in Histobone scaffold (engineered synthetic bone substitute) for the treatment of bone defects caused by pseudoarthrosis, infections, resections, ...
Manufactured by:Biomedal S.L. based inSevilla, SPAIN
iVYCHECK GIP Urine is an immunochromatographic test designed to detect Gluten Immunogenic Peptides (GIP) in urine samples. To perform the test, a urine sample is mixed with a conditioning solution. Then, the detection step is based on the reaction of the GIP present in the sample with the coloured antibody conjugates present in the strip. When there is GIP in the sample due to gluten intake, a ...
Manufactured by:Biomedal S.L. based inSevilla, SPAIN
iVYLISA GIP Stool is a quantitative Sandwich ELISA designed to detectandquantify Gluten Immunogenic Peptides (GIP) in stool samples. The capturing and detecting antibody is the G12, which is designed against the 33-mer peptide and which recognizes it and other equivalent immunotoxic peptides 14. These antibodies have shown great sensitivity and specificity when they have been used to certify ...
Manufactured by:Histocell based inDerio, SPAIN
The HC016 platform is a treatment of acute lung injury, based on mesenchymal stem cells derived from adipose tissue (ASC), improved with proprietary Histocell ...
Manufactured by:Theriva Biologics, Inc., formely known as ynthetic Biologics, Inc. based inRockville, MARYLAND (USA)
Building on the clinical advancement of VCN-01, Theriva developed the Albumin Shield™ technology to protect oncolytic viruses from circulating anti-oncolytic virus antibodies after systemic administration. We anticipate that this technology will enable multiple oncolytic virus doses to be administered in therapeutic cycles to treat particularly refractory ...
by:STALICLA based inGeneve, SWITZERLAND
STALICLA’s precision medicine approach begins with ‘endophenotyping’-identifying the nonbehavioral physical and molecular presentations of NDDs and characterizing subgroups of patients with similar disease signatures. DEPI matches these patient subgroups with an NDD-targeted drug (or drug combination) by integrating and analyzing large omics data sets, including genomics, ...
by:CZ VACCINES based inO Porriño, Pontevedra, SPAIN
CZ Vaccines offers CDMO biotech to third companies and holds EU GMP certificate. CZ Vaccines is an ideal partner for any organization intending to advance their biological products from the development stage until clinical testing phase I and II as well as to production on a commercial scale. ...
Manufactured by:Marsi Bionics, S.L. based inRivas-Vaciamadrid, SPAIN
First robotic knee orthosis with adaptable stiffness, which interprets the needs of the patient and provides the strength, mobility and stability necessary to be able to walk. Sensors provides biomechanical analysis of the patient’s gait and works to improve ...
Manufactured by:Adamo Robot based inMadrid, SPAIN
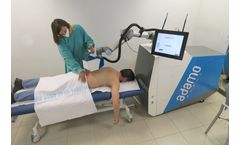
The world’s first collaborative robot that treats MSDs with compressed air. For Rehabilitation Centers and Hospitals – Clinics or Sport Clubs. ADAMO is a robotic medical device capable of aiding in the diagnosis of back pain injuries and delivering contactless treatment using temperature-controlled pressurized air. ADAMO can help reduce patient recovery times, waiting lists, and ...