- Home
- Equipment
- switzerland
- in vitro diagnostic
Refine by
In Vitro Diagnostic Equipment Supplied In Switzerland
11 equipment items found
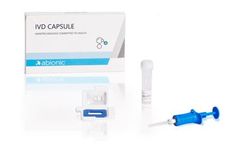
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
Forcing molecules into a nanochannel, limiting the travel distance to a few hundred nanometres and reducing incubation time to 2 ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the determination of Pancreatic Stone Protein (PSP) in blood for the calculation of the cSOFA score at the point of care. The cSOFA score is used to assess the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE COVID-19 is a rapid, single-use in vitro diagnostic test that measures the presence of a specific SARS-CoV-2 viral antigen in saliva. Abionic’s COVID-19 saliva test allows for ultra-fast screening of COVID-19 patients at the point-of-care with lab quality ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Ferritin is a rapid, single-use in vitro diagnostic test for the quantitative measurement of ferritin in capillary and venous whole blood, to aid blood diagnostic testing for iron deficiency in ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in ...
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
Elecsys® Total-Tau CSF (tTau) is an in vitro diagnostic immunoassay intended for the quantitative determination of the total tau protein concentration in human cerebrospinal fluid ...
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
The cobas® Factor II and Factor V Test is an in vitro diagnostic device that uses real-time Polymerase Chain Reaction (PCR) for the detection and genotyping of the human Factor II (Prothrombin) G20210A mutation and the human Factor V Leiden G1691A mutation, from genomic DNA obtained from K2EDTA whole blood specimens, as an aid in diagnosis of patients with ...
Manufactured by:Agappe Diagnostics Switzerland GmbH based inCham, SWITZERLAND
Mispa Count X Plus is a quantitative Auto Hematology Analyzer and 3-part differential counter for in Vitro Diagnostic Use in clinical laboratories. The purpose of this analyzer is to identify the normal patient, with all normal system-generated parameters, and to flag or identify patient ...
by:GenomSys based inPréverenges, SWITZERLAND
GenomSys Codec Suite is a collection of software tools to process genomic data compliant with ISO/IEC-23092 genomic data standard (MPEG-G). It is CE marked as an in-Vitro Diagnostic Medical Device according to 98/79/CE directive and hence approved for clinical-grade diagnostic purposes. The tools enable organizations to implement the standard ...
by:GenomSys based inPréverenges, SWITZERLAND
GenomSys Variant Analyzer is a CE-marked platform natively operating on MPEG-G genomic data format that enables accurate variants identification, annotation, and interpretation (SNVs, indels, CNVs). Based on state-of-the-art pipelines and leveraging MPEG-G-specific features, GenomSys Variant Analyzer supports professionals in the diagnostic process and selecting treatment options. Our Variant ...