- Home
- Equipment
- united kingdom
- iso 13485
Refine by
Iso 13485 Equipment Supplied In United Kingdom
22 equipment items found
Manufactured by:Luxfer MEL Technologies based inManchester, UNITED KINGDOM
Our development and manufacture is the ISO 13485 quality system, this is a requirement for future medical device regulations. Our dedicated facility offers high quality material and process control, this lowers customers risk. We can support programmes from kg to tonne; scalability and capability to support programmes through to success. ...
Manufactured by:Bio Molecular Systems based inUpper Coomera, AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
Manufactured by:Westfield Medical Ltd based inRadstock, UNITED KINGDOM
Coated paper is recommended when fibre free peels are required and/or when sealing to ridged or semi-ridged trays. Coated papers are suitable for ETO and Irradiation sterilisation. See below for stock material options and standard combinations for top and bottoms ...
Manufactured by:Westfield Medical Ltd based inRadstock, UNITED KINGDOM
Direct Seal paper is recommended when high tear/puncture resistance is not so critical. Direct seal papers are suitable for ETO, Irradiation and Steam ...
by:Biofortuna Limited based inDeeside, UNITED KINGDOM
Customised lyophilised beads. Single dose accuracy, manufactured at scale. Lyophilised reagent beads are customisable spheres containing an accurately measured single dose of formulation. Offering all of the stabilisation, transportation and storage benefits of traditional lyophilised formats, beads present a range of additional benefits designed to deliver greater accuracy, improve efficiency ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
Quality of blow indicator. 600 test memory. Suitable for use with Bacterial Viral Filters (BVF™). Compliant with: ATS/ERS 2019 and ISO 23747 / 26782 / 13485. Large, easy to read display. ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
Ideal for monitoring PEF and FEV1 at home, work, in clinical trials, in primary or secondary ...
Manufactured by:Medovate based inGirton, UNITED KINGDOM
Regional anaesthesia procedures require two operators - an anaesthetist who holds an ultrasound scanner and uses this to guide the needle tip placement and a second operator to inject the anaesthetic solution at a required ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
Full featured desktop spirometry complete with Spirotrac software to integrate with your PC, laptop or tablet. The next generation Pneumotrac™ Spirometer combined with our new and improved Spirotrac® software is a powerful and flexible respiratory diagnostic device. Improved sleek design with no moving parts for optimal robustness. The new Pneumotrac™ has a reduced desktop ...
Manufactured by:Speciality Fibres and Materials Ltd. (SFM) based inCoventry, UNITED KINGDOM
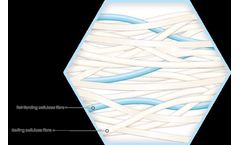
Optimal fibre spacing for increased absorbency. Our Calcium Alginates range can be supplied in roll form, slit reels or cut pieces, packaged and sterile. various GSM available. High absorbency, High conformability, Strong dressing, Reduced fibre shed, various GSM and cut sizes available on request, Biodegradable material. ...
Manufactured by:Speciality Fibres and Materials Ltd. (SFM) based inCoventry, UNITED KINGDOM
Optimal fibre spacing for increased absorbency. Our Carboxymethyl cellulose (CMC) and Cellulose Ethyl Sulfonate (CES) range can be supplied in roll form, slit reels or cut pieces, packaged and sterile. Various GSM available. CE marked and FDA approved. Antibacterial and antimicrobial modified cellulose options ...
Manufactured by:Renishaw plc based inGloucestershire, UNITED KINGDOM
The neuromate system offers you the possibility to develop innovative and productive ways of ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
Quality of blow indicator. 600 test memory. Suitable for use in clinic with Bacterial Viral Filters (BVF™). Compliant with: ATS/ERS 2019 and ISO 23747 / 26782 / 13485 Large, easy to read display. ...
Manufactured by:Speciality Fibres and Materials Ltd. (SFM) based inCoventry, UNITED KINGDOM
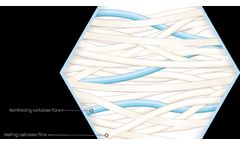
A reinforced alginate dressing with high wet tensile strength for easy removal and high absorbency. A patented blend of alginate fibre and cellulose fibre which provides structural integrity, high tensile strength and high absorbency. Our reinforced alginate range can be supplied in roll form, slit reels or cut pieces, packaged and sterile. various GSM ...
Manufactured by:Speciality Fibres and Materials Ltd. (SFM) based inCoventry, UNITED KINGDOM
Effective against bacteria such as E. Coli, Staphylococcus Aureus and Yeast (Candida Albicans). Our Silver Calcium Alginates fabric can be supplied in roll form, slit reels or cut pieces, packaged and sterile. various GSM available. High absorbency, High conformability, Antibacterial performance*, Strong dressing, Reduced fibre shed, various GSM and cut sizes available on request, Biodegradable ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
Highly portable lightweight all-in-one desktop spirometer and printer. Easy to use and no PC required. Designed for testing on the go, remotely or in a clinic, the Alpha is our ultimate desktop spirometry solution. The new Alpha encompasses compliance with the ATS/ERS 2019 spirometry guidelines, multiple testing options, quick loading integrated thermal printer and direct links to Vitalograph ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
The COMPACT Medical Workstation forms the foundation of our PFT solutions and is a validated medical device for clinic use, with a user friendly interface and integral spirometer. The device hosts questionnaires and centralizes data from a range of pulmonary function tests and other medical ...
Manufactured by:Mi3 Limited based inLancashire, UNITED KINGDOM
As a medical device manufacturing company (CMO), the experts at Mi3 support the design and manufacture of your medical devices, equipment and other medical products. Our purpose built assembly clean-room facilities ensure all medical device manufacturing and medical device assembly is validated and approved to Class 7. Mi3’s clean room facilities: Our clean room is designed for precision ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
AcuPebble RE100 is a wearable device that fundamentally improves the measurement of respiratory biosignals. It provides more accurate data and enables the continuous monitoring of patients at home in clinical trials and post-market ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
Clinically validated: AcuPebble SA100 automated sleep apnea test was validated in a powered clinical trial at the NHS Royal Free London hospital. Validated sleep study result equivalent to ambulatory gold-standard (multi-channel polygraphy followed by manual specialist interpretation). Validated result based on AHI and ODI for both 3% and 4% desaturation criteria with high accuracy (94% PPV, 98% ...