- Home
- Equipment
- usa california
- circulating dna
Show results for
Refine by
Circulating Dna Equipment Supplied In Usa California
9 equipment items found
Manufactured by:Guardant Health, Inc. based inRedwood City, CALIFORNIA (USA)
Our Guardant Reveal™ test is the first blood-only test that detects residual and recurrent disease in two weeks, without the need for a tissue biopsy. The test detects circulating tumor DNA (ctDNA) in blood after surgery to identify patients with residual disease who may benefit most from adjuvant therapy. Our first indication is early-stage colorectal ...
Manufactured by:Twist Bioscience based inSouth San Francisco, CALIFORNIA (USA)
NGS-based liquid biopsy is a rapidly evolving application that requires accurate and precise reference standards. The Twist Pan-cancer Reference Standard is a high-quality, standardized control for use in the research and development of NGS-based liquid biopsy assays. This reference standard is ideal for establishing the analytical limit of detection (LoD) for specific cancer variants and as a ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Lung cancer remains a leading cause of death from cancer globally, due to its high incidence and late-stage diagnosis. Most patients are currently diagnosed with late-stage cancer, though research has shown 5-year survival rates are up to 10 times greater when disease is detected early. Annual imaging (low-dose CT scans) is recommended for screening individuals with highest risk, but less than ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Liquid biopsies are a new powerful tool in cancer diagnostics that is transforming patient healthcare. Body fluids such as blood or urine are obtained from the patient and analyzed for the presence of circulating tumor DNA which is shed from the cancer. This DNA can tell if cancer is present, its type, effective medications, and more. Liquid ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
Biocept’s Target Selector™ molecular assay kits detect key oncogene mutations through the analysis of both Formalin-Fixed Paraffin-Embedded (FFPE) tissue gained from surgical biopsies as well as circulating tumor DNA (ctDNA) gained from blood-based liquid biopsies. The EGFR pathway can include mutations that are among the most frequently evaluated ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
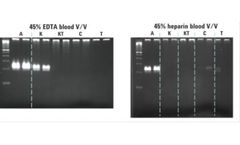
As healthy and diseased tissue interact with the bloodstream, they release both cellular and genetic material into the circulation. These materials are referred to as “cell-free” DNA (cfDNA), and circulating tumor cells (CTCs). Conventional blood collection tubes contain only anti-coagulants, designed to prevent blood clotting. Over ...
by:BillionToOne Inc. based inMenlo Park, CALIFORNIA (USA)
Our molecular counting platform can help quantify nearly undetectable DNA changes, unlocking improvements to non-invasive prenatal screening and liquid ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Signatera is a personalized, tumor-informed assay optimized to detect circulating tumor DNA (ctDNA) for molecular residual disease (MRD) assessment and recurrence monitoring for patients previously diagnosed with cancer, with broad utility for cancer ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureDirect Blood PCR Kit enables direct DNA amplification from many sample types including: liquid blood, dried blood on paper, serum, and plasma. Inhibitor resistance and throughput, common PCR challenges associated with blood samples, are averted through the activity of a newly engineered Taq. Your workflow is streamlined by eliminating the cumbersome DNA extraction step. SureDirect Blood ...