- Home
- Equipment
- usa california
- healed pmma bone
Refine by
Healed Pmma Bone Equipment Supplied In Usa California
16 equipment items found
Manufactured by:Advanced Orthopaedic Solutions based inTorrance, CALIFORNIA (USA)
The AOS 7.0mm Cannulated Screw System is designed to optimize surgical outcomes while simplifying procedures. This system presents to the surgeon the optimal choice of implants, instruments, and disposables for all indicated hip, pelvis, and extremity fracture treatments. The 7.0mm Cannulated Screws offer stable fracture fixation with minimal trauma to vascular supply, providing interfragmentary ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
The Iso-Elastic property of the polymer SuperCable provides dynamic compression across the sternal halves to better accommodate the physiologic loads acting on the healing sternum. This Iso-elastic property also acts as a shock absorber on the sternum, helping to neutralize loads caused by coughing, sneezing and patient movement, thus reducing the chance of cut-through and loss of fixation. ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
The osteoSPAN advanced bone graft product is available in a block form (in addition to granules) that was specifically designed for posterolateral spine fusion. The osteoSPAN Fusion Kit is based on the same TrelCor technology as the osteoSPAN Granules. The Fusion Kit consists of two TrelCor blocks packaged together. The osteoSPAN Fusion Kit is intended to be mixed with autograft for use as a bone ...
Manufactured by:Hand Biomechanics Lab, Inc. based inSacramento, CALIFORNIA (USA)
The PIP Fix is our external fixator for treating dorsal fracture dislocations of the PIP joints of the fingers providing palmar translation and adjustable length restoration. Indicated for the treatment of unstable dorsal fracture dislocations of the proximal interphalangeal (PIP) joint of the fingers in which external fixation as provided by the PIP Fix alone is sufficient to obtain and maintain ...
Manufactured by:Starch Medical, Inc. based inSan Jose, CALIFORNIA (USA)
SealFoam® Absorbable Polysaccharide Hemostat is a medical device composed of AMP® (absorbable modified polymer) particles which are very hydrophilic, biocompatible, non-pyrogenic and derived from purified plant starch.The device contains no human or animal components. SealFoam® is a naturally adhesive absorbable hemostatic pad designed to control bleeding during surgical procedures or ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
Viable Cell Population. An average of 1.5 million viable osteogenic cell population per cc of matrix are preserved via a non-DMSO cryoprotectant2. Endogenous bone cells remain attached via advanced processing techniques. Viable endogenous bone cells support osteogenic healing ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Strip Collagen Enhanced Bone Grafting Product is the latest addition to the Biogennix bone grafting product family. Developed with Biogennix proprietary TrelCor technology, Agilon Strip consists of resorbable, osteoconductive TrelCor granules densely packed in a matrix of type 1 bovine ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Moldable bone grafting product is the latest moldable grafting solution in Biogennix’ advanced bone graft product line. Developed with Biogennix proprietary TrelCor® technology, Agilon Moldable consists of a suspension of 1-2mm TrelCor granules in a rapidly absorbing, organic binder blended with type-1 ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Biogennix’s flagship product brand, Morpheus, is an advanced bone graft product consisting of a moldable suspension of 1-2mm TrelCor granules in a rapidly absorbing, organic binder. Based on TrelCor technology, the granules provide enhanced bone formation due to the combination of a nanocrystalline surface with an HCA composition and interconnected pore structure that emulate human ...
Manufactured by:Glidewell Direct based inNewport Beach, CALIFORNIA (USA)
Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant is a contemporary dental implant tailored to the demands of modern implant dentistry. Precisely engineered to meet the exacting requirements of implant pioneer Dr. Jack Hahn, this advanced system addresses today's clinical challenges with a blend of time-tested features and innovation. Excellent primary ...
Manufactured by:Glidewell Direct based inNewport Beach, CALIFORNIA (USA)
Combining decades of clinical experience with cutting-edge design, the Hahn Tapered Implant is a contemporary dental implant tailored to the demands of modern implant dentistry. Precisely engineered to meet the exacting requirements of implant pioneer Dr. Jack Hahn, this advanced system addresses today's clinical challenges with a blend of time-tested features and innovation. Excellent primary ...
Manufactured by:Glidewell Direct based inNewport Beach, CALIFORNIA (USA)
The Hahn Tapered Implant Surgical Kit allows the clinician to easily organize, store, and transport the surgical tooling of the Hahn Tapered Implant System. The autoclavable, color-coded surgical kit clearly maps the drilling protocol for each implant. Drills are arranged from left to right and top to bottom in order of increasing diameter / bone density, with color-coded bands on the shaping ...
Manufactured by:Glidewell Direct based inNewport Beach, CALIFORNIA (USA)
The Hahn™ Tapered Implant Guided Surgical Kit allows the clinician to easily organize, store, and transport the surgical tooling of the Hahn Tapered Implant Guided Surgery System. The autoclavable, color-coded surgical kit clearly maps the drilling protocol for each implant. Drills are arranged from left to right and top to bottom in order of increasing diameter / bone density, with ...
Manufactured by:Ankasa Regenerative Therapeutics based inLa Jolla, CALIFORNIA (USA)
Stem cell renewal and maintenance and tissue regeneration are critical for normal health and well-being. Wnt pathway interactions are crucial to maintenance of these processes. Ankasa is the first to produce human Wnt proteins in a manufacturing setting suitable for use in humans. Our first clinical approach will be the demonstration of improved outcomes after spinal fusion surgeries. Our product ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
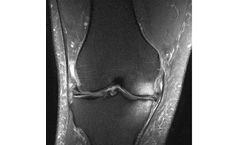
Bone Marrow Lesions (BML) are MRI-visible defects in the subchondral bone. BML can only be seen on fat-suppressed MRI sequences (T2FS, PDFS, etc.) where they appear as a hazy white area against the background of darker bone. Pathologists have shown that BML represent a healing response to trauma such as micro trabecular fractures of the subchondral ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft substitute that resorbs and is replaced with bone during the healing ...