Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Healed Pmma Bone Equipment Supplied In Usa
60 equipment items found
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
Nitinol is a metal alloy developed by the U.S. Navy. It got its name because it is composed of essentially of 50% nickel and 50% titanium. Nitinol is frequently called “memory metal” because it is super-elastic and has shape-memory, which means it can be twisted or stressed and will return to its original ...
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
A simple, yet comprehensive, system designed for stabilization and rigid fixation. The RibFix Blu Fixation System is designed to offer surgeons simplicity and ease of use when approaching rib fracture repair. RibFix Blu plates are engineered to match the natural shape of the ribs, offering stability for direct bone healing while considering the natural movement of the chest wall. Pre-contoured ...
Manufactured by:MPR Orthopedics based inEagan, MINNESOTA (USA)
This improved classic Alvarado positioner system allows ready access to the knee as well as selective adjustments to the degree of rotation. Positioner’s boot, or splint assembly, is constructed of aluminum. System also includes IMPROVED fully welded base plate and no screws. Made in the USA. ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
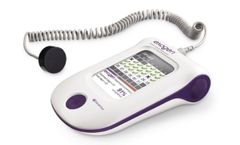
The PhysioStim device provides a safe, non-invasive option for treating fractures that are difficult to heal. With an overall clinical success rate of 80 percent (up to 88 percent for fracture gaps less than 3mm), PhysioStim devices have high success rates for treating nonunion fractures. The device assists in fracture healing by delivering a pulsed electromagnetic field (PEMF) signal to the ...
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
Our proprietary Staple Compression Plates™ (SCPs) are the only plates which utilize a staple instead of a lag screw to provide compression. This gives three important advantages: Gap Recovery: Compressing the fusing joint if there is bone resorption, Apposition: Provides full bone surface contact, Speed: Reduces OR time by up to an hour, SCP gap recovery, apposition and speed (GAS) all work ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new bone. ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
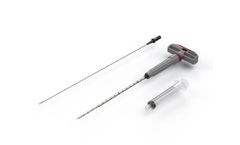
The CER-ORTHO-M device incorporates patented technology that limits peripheral blood dilution and eliminates the need for centrifugation. The length makes it ergonomically friendly for accessing the iliac crest and can be advanced wither manually or with a mallet. This device is often used in conjunction with the CER-BN-84 bone dowel harvesting device and CF-G10 bone graft granules. Combining ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The CER-LA-M device incorporates patented technology that limits peripheral blood dilution and eliminates the need for centrifugation. Its length makes it ergonomically friendly for accessing deeper locations needed for minimally invasive spine procedures. The T-Handle allows it to be advanced either manually or with a mallet. This device is often used in conjunction with the CER-BN-116 bone ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
Data Driven Performance - In Vivo Study Results; Bioactive glass has typically been used in a particulate form consisting of irregular particles and a broad size range (32-710 um). Instead of creating a bone graft with these "off-the-shelf" particles, Synergy utilized a unique spherical particle shape and conducted an in vivo optimization study to determine the size range that provided the best ...
by:In2Bones Global based inMemphis, TENNESSEE (USA)
Tribio™ Backfill Plugs are a proprietary material delivered sterile and ready for use in any bony voids or gaps in the skeletal system. Consisting of hydroxyapatite, tricalcium phosphate and bioactive glass in a collagen matrix, the Backfill Plugs are sized to easily fit in surgically created osseous defects; 5.5mm dia. (for 6mm void) and 7.5mm dia. (for 8mm void) by 40mm in length. The ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
BioSphere Flex SP is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere® Technology, BioSphere® Flex SP is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate carrier. ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
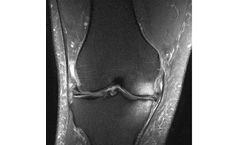
Bone Marrow Lesions (BML) are MRI-visible defects in the subchondral bone. BML can only be seen on fat-suppressed MRI sequences (T2FS, PDFS, etc.) where they appear as a hazy white area against the background of darker bone. Pathologists have shown that BML represent a healing response to trauma such as micro trabecular fractures of the subchondral ...
Manufactured by:Zetagen Therapeutics Inc. based inSyracuse, NEW YORK (USA)
ZetaFuse is the first synthetic, small molecule, osteo-inductive biologic, to safely aid bone healing of spinal fusions. The U.S. Food and Drug Administration (FDA) has awarded ZetaFuse Breakthrough Device Designation[1] for the complete spinal column. ZetaFuse is driven by the activation of a novel molecular pathway, by leveraging this patented mechanism, to directly turn stem cells into ...
Manufactured by:Bioventus Inc. based inDurham, NORTH CAROLINA (USA)
Using safe, painless ultrasound waves to activate cells near the site of the break, the EXOGEN Ultrasound Bone Healing System stimulates the body’s natural healing process1, helping fractured bones mend. When treating fractures, EXOGEN may be a cost-effective alternative to more invasive ...
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The CER-EXT-M has a profile that makes it ideal for use in extremity bones. The enhanced features of the tip of the stylet and cannula facilitate penetrating harder cortical bone including the distal tibia. Its shorter length makes it ergonomically friendly for working in extremity locations and it incorporates patented technology that limits peripheral blood dilution and eliminates the need for ...
by:STERIS based inMentor, OHIO (USA)
Xorption® Cages are made from proven, high quality bio-absorbable polymer that mimics and promotes natural bone healing. ...
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
The dynaBunion® Lapidus system gives surgeons the instruments and implants to correct bunions in 4 dimensions (4D) through a minimal-incision. A traditional Lapidus surgery requires surgeons to make a large incision and manually hold several small bones in 4D simultaneously while trying to attach a surgical plate. Frequently, surgeons wish for an “extra set of hands”. The ...
Manufactured by:Advanced Orthopaedic Solutions based inTorrance, CALIFORNIA (USA)
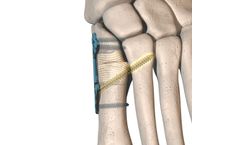
The AOS 7.0mm Cannulated Screw System is designed to optimize surgical outcomes while simplifying procedures. This system presents to the surgeon the optimal choice of implants, instruments, and disposables for all indicated hip, pelvis, and extremity fracture treatments. The 7.0mm Cannulated Screws offer stable fracture fixation with minimal trauma to vascular supply, providing interfragmentary ...
Manufactured by:Surgentec LLC based inBoca Raton, FLORIDA (USA)
OsteoFlo NanoPutty is the world’s first, and only quadphasic synthetic bone graft putty with nano-surface technology that allows for advanced bone healing. OsteoFlo® NanoPutty® has been designed to provide true nanobiology with an optimal resorption profile and superior handling characteristics. This novel synthetic bone graft putty is comprised of particles featuring four different ...