- Home
- Equipment
- usa california
- igg igm combined test kit
Refine by
Igg Igm Combined Test Kit Equipment Supplied In Usa California
7 equipment items found
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Qualitative detection of Hepatitis B Core Antibody (HBcAb) in serum or ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
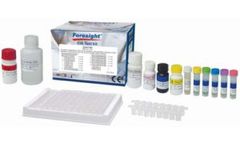
Qualitative and quantitative detection of IgG antibodies to Helicobacter pylori (H. pylori) in serum or ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
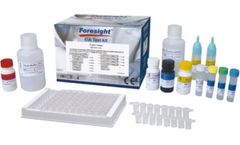
Qualitative and quantitative detection of Helicobacter pylori (H. pylori) Antigen in ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
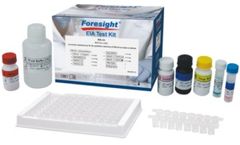
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
The SARS-CoV-2 IgG and IgM EIA Test Kits are qualitative enzyme immunoassays for the detection of IgG or IgM antibodies to SARS-CoV-2 in human serum or ...
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The test can be used for a variety of samples including serum, plasma, whole blood, dry blood spot, and saliva in a lab facility. It can be used to assess IgG and IgM levels in a sample with 100% sensitivity and 99.87% specificity without interferences from other diseases. Avidity pGOLD™ assay can tell if a COVID-19 infection is recent or remote greater than 6 months ...