- Home
- Equipment
- usa massachusetts
- gastrointestinal
Show results for
Refine by
Gastrointestinal Equipment Supplied In Usa Massachusetts
17 equipment items found
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
CRE PRO, CRE RX, CRE Fixed, and CRE Wireguided Balloon Dilatation Catheters provide consistent performance for balloon endoscopy for optimal control, efficiency and performance. Both CRE RX Biliary and PRO Wireguided Catheters are indicated for use in the removal of difficult biliary stones (Dilatation Assisted Stone Extraction, ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
Available in a wide range of sizes, the Hurricane™ RX Biliary Balloon Dilatation Catheter is designed to reduce resistance and increase pushability when crossing ...
Manufactured by:Universal Diagnostics SL based inSeville, SPAIN
We are developing Signal-X, a multi-cancer platform able to detect various types of high-burden cancers with high sensitivity and tissue-of-origin specificity. ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
FIN-211 is an orally administered, Enriched Consortia product candidate designed to address both the gastrointestinal (GI) and behavioral symptoms of ASD. ASD is a behaviorally defined condition characterized by difficulties with interpersonal interaction, communication, and repetitive or restrictive patterns of behavior and activities. A subset of individuals with ASD experience ...
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Energy-sealing devices, dissection devices, and RF therapy devices for procedural areas including: Gastrointestinal, Urologic, Prostate care, Women’s health, Phaco needles and lens ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-155 is an investigational, oral, rationally designed, fermented microbiome therapeutic designed to reduce the incidence of gastrointestinal infections, bacteremia, and graft versus host disease (GvHD) in immunocompromised patients, including patients receiving allogeneic hematopoietic stem cell transplantation ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
In collaboration with Takeda Pharmaceuticals, we are developing FIN-524 and FIN-525, orally administered, Targeted Consortia product candidates designed for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD), respectively. UC and CD are the two most common types of IBD, an autoimmune condition that causes inflammation of the gastrointestinal (GI) ...
by:Vertex Pharmaceuticals based inBoston, MASSACHUSETTS (USA)
Cystic fibrosis (CF) is a rare, chronic and life-shortening genetic disease. It’s a progressive, multi-system disease that affects the lungs, liver, gastrointestinal tract, pancreas, sinuses, sweat glands and reproductive tract. In the lungs, this leads to the buildup of abnormally thick, sticky mucus that can cause chronic lung infections and inflammation, resulting in ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A new generation of multifunctional oncolytic virus, TG6002 has been designed to combine the mechanism of oncolysis (targeted destruction of the cancer cell) with the production of chemotherapy (5-FU), directly into the ...
by:Kephera Diagnostics, LLC based inFramingham, MASSACHUSETTS (USA)
With support from a National Institutes of Health grant, Kephera is developing a new point-of-care test for Chagas disease. Kephera scientists have identified and are purifying parasite components which promise to yield a highly sensitive and specific test. Together with our academic collaborators in the U.S. and Latin America, we are evaluating the performance of the test as it is developed in ...
Manufactured by:SQ Innovation, Inc. based inBurlington, MASSACHUSETTS (USA)
Lasix ONYU is an advanced prescription drug-device combination designed specifically for the treatment of edema in adults suffering from chronic heart failure. This innovative product represents a shift from traditional oral and intravenous methods by introducing a high-concentration formulation of furosemide for subcutaneous infusion. Each prefilled cartridge within the device delivers 80 mg of ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-287 is an investigational, oral, biologically-derived microbiome therapeutic for the treatment of ulcerative colitis (UC). SER-287 is a consortium of multiple bacterial spores manufactured by fractionating and purifying targeted bacteria from stool of healthy human donors. SER-287 has been granted Fast Track designation and Orphan Drug designation by the FDA. A completed SER-287 Phase 1b ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
Our lead product candidate, CP101, is an orally administered, Complete Consortia product candidate initially targeting the prevention of recurrent CDI. The Centers for Disease Control and Prevention considers CDI to be one of the top three most urgent antibiotic resistant threats and the most common cause of healthcare associated infection in the United ...
Manufactured by:Senzime AB Uppsala based inUppsala, SWEDEN
OnZurf® Probe is a sterile single use product based on micro-dialysis technology that enables continuous sampling from gastro-intestinal organs such as esophagus, stomach and liver. OnZurf® Probe is a unique micro-dialysis catheter for clinical applications, which is fixed to the surface of the organ without penetrating the tissue and causing unnecessary organ stress. Experimental studies ...
by:TISSIUM based inParis, FRANCE
Our proprietary biomorphic programmable polymers enable tissue reconstruction and can be adapted for use in multiple clinical ...
Manufactured by:GI Dynamics, Inc. based inBoston, MASSACHUSETTS (USA)
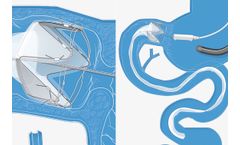
EndoBarrier is an endoscopically delivered medical device intended to help restore healthy blood sugar levels and reduce weight. EndoBarrier is an investigational medical device for people living with uncontrolled type 2 diabetes and obesity that is sought to bridge the gap between partially effective medications, insulin injections, and irreversible ...
Manufactured by:Eagle Pharmaceuticals, Inc. based inWoodcliff Lake, NEW JERSEY (USA)
Indication: PEMFEXY is indicated in combination with cisplatin for the initial treatment of patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC). PEMFEXY is indicated as a single agent for the maintenance treatment of patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) whose disease has not progressed after four ...