- Home
- Equipment
- usa massachusetts
- haematopoietic stem cells
Refine by
Haematopoietic Stem Cells Equipment Supplied In Usa Massachusetts
8 equipment items found
Manufactured by:Editas Medicine based inCambridge, MASSACHUSETTS (USA)
We are pursuing a number of diseases using an in vivo editing approach, focusing on hematopoietic stem cells and other tissue types, and we are excited about the progress we’ve ...
Manufactured by:Sana Biotechnology based inSeattle, WASHINGTON (USA)
Hemoglobinopathies: Sickle cell disease, ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
Our first multi-targeted Treatment System is comprised of VOR33-CLL1 multiplex-edited eHSC therapy and VCAR33-CLL1 multi-specific CAR-T therapy. Knocking out CD33 and CLL-1 through gene editing offers a promising new approach to treating patients with AML using our novel eHSC platform. Our research demonstrates that multiplex genome editing of allogeneic hematopoietic stem cells may represent ...
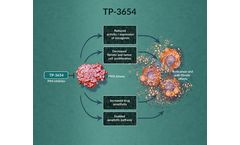
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
TP-3654 is an oral investigational inhibitor of PIM kinases, which has potential antitumor and anti-fibrotic effects through multiple pathways, including induction of ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
VOR33 is our lead eHSC product candidate designed to replace the standard of care in transplant settings. Once the VOR33 cells have engrafted, we believe that patients can be treated with anti-CD33 therapies, such as Mylotarg or VCAR33, with limited on-target toxicity, leading to durable anti-tumor activity and potential cures. In preclinical studies, we have observed that the removal of CD33 ...
Manufactured by:Editas Medicine based inCambridge, MASSACHUSETTS (USA)
EDIT-301 is an experimental cell therapy medicine under investigation for the treatment of severe sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). EDIT-301 consists of patient-derived CD34+ hematopoietic stem and progenitor cells edited at the gamma globin gene (HBG1 and HBG2) promoters, where naturally occurring fetal hemoglobin (HbF) inducing mutations reside, by a ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-155 is an investigational, oral, rationally designed, fermented microbiome therapeutic designed to reduce the incidence of gastrointestinal infections, bacteremia, and graft versus host disease (GvHD) in immunocompromised patients, including patients receiving allogeneic hematopoietic stem cell transplantation ...
by:Intellia Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
We plan to initiate patient screening for a first-in-human Phase 1 study of NTLA-5001, a WT1-directed TCR T cell therapy for the treatment of AML, by the end of 2021. Our first ex vivo development candidate seeks to treat acute myeloid leukemia (AML) by engineering autologous T cell receptors (TCR) directed towards the Wilms’ Tumor 1 (WT1) antigen, an over-expressed protein that is often ...