- Home
- Equipment
- usa new jersey
- in vitro diagnostic
Refine by
In Vitro Diagnostic Equipment Supplied In Usa New Jersey
10 equipment items found
Manufactured by:SEQENS Group based inÉcully, FRANCE
Located in Limoges (France), SEQENS IVD manufactures fractionated blood products for the life sciences and IVD industries. Thanks to over 25 years of experience, the company has developed a considerable expertise in blood fractionation, extraction and purification. SEQENS IVD is a part of the French group SEQENS since ...
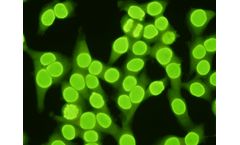
Manufactured by:Scimedx Corporation based inDover, NEW JERSEY (USA)
Indirect Immunofluorescence Assay is a qualitative or semi-quantitative assay for the in vitro diagnostic detection of Anti-nuclear Antibodies in human serum on human epithelial cells (HEp2). The test is intended as an aid in the diagnosis of SLE and connective tissue ...
Manufactured by:ZEUS Scientific, Inc. based inBranchburg, NEW JERSEY (USA)
This test system is intended to be used as an aid in the diagnosis of EBV-associated infectious mononucleosis and to provide epidemiological information on the diseases caused by Epstein-Barr Virus. This test for in vitro diagnostic ...
Manufactured by:ZEUS Scientific, Inc. based inBranchburg, NEW JERSEY (USA)
This test system is intended to be used as an aid in the diagnosis of EBV-associated infectious mononucleosis and to provide epidemiological information on the diseases caused by Epstein-Barr virus. This test for in vitro diagnostic ...
Manufactured by:ZEUS Scientific, Inc. based inBranchburg, NEW JERSEY (USA)
The test system is intended to be used as an aid in the diagnosis of diseases caused by the exposure to Toxoplasma gondii, Rubella, Cytomegalovirus and Herpes Simplex 1 and 2 and/or to provide epidemiological information on the associated disease. This test for in vitro diagnostic ...
Manufactured by:Immunostics, Inc based inEatontown, NEW JERSEY (USA)
ALFIS-3 tests are used for measuring the concentration of various analytes in human blood, plasma, and serum. ALFIS-3 tests are in vitro diagnostic tests intended for screening, monitoring and/or routine physical examination in centralized laboratories of hospitals and physician ...
Manufactured by:Beta Lifescience based inPaoli, NEW JERSEY (USA)
The Anti SARS-COV2 / 2019-nCoV NP mAb (IgG&IgM Positive Control) product contains two isotype antibody clone Human IgG and IgM, which can recognizes2019-nCoV Nucleocapsid ...
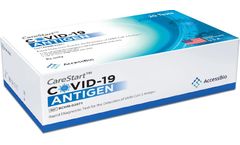
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 MDx RT-PCR is a real-time reverse transcription polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in respiratory specimens (such as nasopharyngeal, oropharyngeal and nasal swabs, and nasopharyngeal wash/aspirate or nasal aspirate) and bronchoalveolar lavage from individuals suspected of COVID-19 by their ...
Manufactured by:Thermo Scientific - LC/MS GC GC/MS based in, MASSACHUSETTS (USA)
Designed to make next-generation sequencing (NGS) accessible to clinical laboratories with all levels of sequencing expertise, the Ion Torrent Genexus Dx Integrated Sequencer delivers an effortless user experience while enabling regulatory ...