- Home
- Equipment
- usa new york
- drug delivery applications
Refine by
Drug Delivery Applications Equipment Supplied In Usa New York
6 equipment items found
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
SIGA has developed TPOXX® Injection, an intravenous (IV) formulation of TPOXX® for those patients who are too sick or unable to swallow oral capsules. SIGA completed clinical trials for the IV formulation with funding from the Biomedical Advanced Research and Development Authority (BARDA). In April 2021, SIGA submitted a New Drug Application (NDA) with the U.S. Food and Drug ...
Manufactured by:Alfa Chemistry based inNY, NEW YORK (USA)
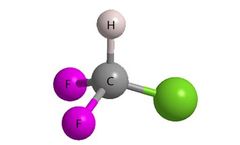
Introducing fluorine atom(s) into bioactive organic compounds has become a leading strategy for drug design and lead optimization. In drug synthesis strategy, fluorine is frequently employed to modify biologically relevant properties such as metabolic stability, basicity, lipophilicity and bioavailability. Additionally, the binding affinity of compounds to biological targets may also improve upon ...
by:Curemark based inRye Brook, NEW YORK (USA)
The U.S. Centers for Disease Control and Prevention estimate that 1 in 68 children in the United States have been diagnosed with Autism. There is currently no approved drug on the market that treats the core symptoms of Autism. At Curemark, we are working to change that. Curemark’s lead drug candidate, CM-AT, has received “Fast Track” designation from the U.S. Food and Drug ...
Manufactured by:Pluristem Therapeutics Inc. based inHaifa, ISRAEL
PLX-R18 cells release a combination of therapeutic proteins in response to a damaged or poorly functioning hematopoietic system; this system creates the blood cells that protect us from infection, uncontrolled bleeding and anemia. PLX-R18 is currently in a Phase I clinical trial to treat incomplete hematopoietic recovery following Hematopoietic Cell Transplantation (HCT) and in development to ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-1300 (recombinant T172R/G173Q double-mutant cocaine esterase 200 mg, IV solution) is being developed under an Investigational New Drug application (IND) for the treatment of cocaine intoxication. It has been designated a Breakthrough Therapy by the FDA. Currently there is no specific pharmacotherapy indicated for cocaine intoxication, a state characterized by acute agitation, hyperthermia, ...
Manufactured by:BlueRock Therapeutics based inCambridge, MASSACHUSETTS (USA)
With our cell+gene platform, BlueRock is aiming to redefine the very way we treat disease, moving from addressing mere symptoms to restoring health lost to cell damage or degeneration. Instead of prescribing a pill to treat tremors in patients with Parkinson’s, we might restore the chemical messengers that were destroyed, addressing tremors at the source. Those who have suffered a heart ...