Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Drug Delivery Applications Equipment Supplied In Usa
48 equipment items found
Manufactured by:NP Medical Inc. based inClinton, MASSACHUSETTS (USA)
The Pressure Activated Valve is used extensively as a "one-way" valve in critical drug delivery applications, and as an "anti-siphon" valve in pump applications. It targets patient applications requiring very specific performance characteristics such as high-pressure rating and narrow specification ranges for key ...
Manufactured by:ALZET Osmotic Pumps based inCupertino, CALIFORNIA (USA)
The iPRECIO programmable pump is an advanced infusion device for drug delivery applications in mice, rats and larger animals. With versatile programmable functions and a refillable reservoir, this implantable infusion pump enables full control over dosing studies and empowers researchers to execute even the most complex dosing protocols, ...
Manufactured by:Tekni-Plex based inWayne, PENNSYLVANIA (USA)
CELLENE™ MC1053 is a high clarity, nontoxic, halogen and phthalate free, flexible thermoplastic elastomer compound developed specifically for extrusion applications in the food and medical industries. All components used in the manufacturing of CELLENE™ MC1053 are sanctioned by the US Food & Drug Administration for food applications under Title 21 of the Code of Federal ...
Manufactured by:RoosterBio, Inc. based inFrederick, MARYLAND (USA)
RoosterCollect™-EV-CC is a standardized chemically defined, and low particulate collection medium for scalable and streamlined extracellular vesicle (EV) collection. RoosterCollect-EV-CC is one component of RoosterBio’s CliniControl™ EV Production System – a bioprocess engine for rapid EV manufacturing for use in cGMP and clinical applications. Each bag of ...
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
megaPOD consists of high bay POD units for oversized equipment. Ballroom configurations are available. RECOMMENDED FOR Any medicinal drug or medical device application (upstream/ downstream processing, formulation, filling, packaging, gene/viral vector ...
Manufactured by:Suvoda based inConshohocken, PENNSYLVANIA (USA)
Overcome the traditional challenges of electronic outcomes data collection and submission for complex clinical ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
SIGA has developed TPOXX® Injection, an intravenous (IV) formulation of TPOXX® for those patients who are too sick or unable to swallow oral capsules. SIGA completed clinical trials for the IV formulation with funding from the Biomedical Advanced Research and Development Authority (BARDA). In April 2021, SIGA submitted a New Drug Application (NDA) with the U.S. Food and Drug ...
Manufactured by:Alfa Chemistry based inNY, NEW YORK (USA)
Introducing fluorine atom(s) into bioactive organic compounds has become a leading strategy for drug design and lead optimization. In drug synthesis strategy, fluorine is frequently employed to modify biologically relevant properties such as metabolic stability, basicity, lipophilicity and bioavailability. Additionally, the binding affinity of compounds to biological targets may also improve upon ...
Manufactured by:Biopharma PEG Scientific Inc based inWatertown, MASSACHUSETTS (USA)
Uses: Applicated in medical research, drug-release, nanotechnology and new materials research, cell culture. In the study of ligand, polypeptide synthesis support, a graft polymer compounds, new materials, and polyethylene glycol-modified functional coatings and other aspects of the active ...
Manufactured by:BioXcel Therapeutics, Inc. based inNew Haven, CONNECTICUT (USA)
BXCL501 is our most advanced neuroscience clinical program. BXCL501 is a proprietary, sublingual film formulation of dexmedetomidine, a selective alpha-2 receptor agonist. It is being investigated for at-home use for the acute treatment of agitation in bipolar and schizophrenia patients, for agitation in Alzheimer’s disease, and as an adjunctive treatment for major depressive ...
Manufactured by:InSphero AG based inSchlieren, SWITZERLAND
When you’re ready, we deliver. InSphero assay-ready spheroid microtissues are engineered and certified for use in critical drug development applications, and validated to ensure uniform size, robust functionality, and reproducible results. We develop 3D InSight™ Microtissues using the most advanced 3D cell culture technologies available to produce highly predictive, fully QC’d ...
Manufactured by:G-CON Manufacturing, Inc. based inCollege Station, TEXAS (USA)
The POD System integrates multiple connected units to accommodate larger footprint installations with suites, airlocks, and corridors. RECOMMENDED FOR Any medicinal drug or medical device application (upstream/ downstream processing, formulation, filling, packaging, gene/viral vector ...
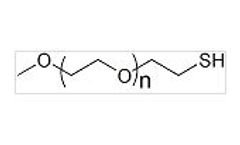
Manufactured by:Biopharma PEG Scientific Inc based inWatertown, MASSACHUSETTS (USA)
Uses: Applicated in medical research, drug-release, nanotechnology and new materials research, cell culture. In the study of ligand, polypeptide synthesis support, a graft polymer compounds, new materials, and polyethylene glycol-modified functional coatings and other aspects of the active compound. Methoxy PEG Thiol (mPEG-SH) is a linear monofunctional PEG, which has a free thiol group at one ...
Manufactured by:Allevi, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Clinically relevant drug-drug interactions (DDI) are a serious concern for any new drug development project. To better support capturing the multiple mechanisms of DDI potential using primary hepatocytes, we now offer Interaction Qualified Human Hepatocytes (Catalog HUCPI) which are characterized for 3 major mechanisms of DDI: transporter activity, enzyme activity, and induction potential, ...
Manufactured by:Elixir Medical Corporation based inMilpitas, CALIFORNIA (USA)
Novolimus is a metabolite of the drug Sirolimus, a novel mTOR inhibitor macrolytic lactone with anti-proliferative and anti-inflammatory properties developed by Elixir Medical. The macrocyclic lactone drugs represent the most widely utilized drug family for drug-eluting stent applications and have an established safety and efficacy ...
by:BICO - The Bio Convergence Company based inBoston, MASSACHUSETTS (USA)
Automated sorting and isolation of single spheroids and organoids. A game-changer in drug screening and other applications where standard 3D models will gradually replace traditional animal models. spheroONE is an innovative single large-particle sorter and dispenser which revolutionizes 3D cellular models handling. Using precision dispensing technology together with advanced image-based sorting ...
Manufactured by:Cingulate based inKansas City, KANSAS (USA)
CTx-1302 utilizes a breakthrough multi-core formulation of dextroamphetamine, a compound approved by the FDA for the treatment of ...
Manufactured by:Elixir Medical Corporation based inMilpitas, CALIFORNIA (USA)
Sirolimus is the most widely known mTOR inhibitor with a well-established safety and effectiveness profile in various treatment applications: Drug-eluting stents, Transplant, Oncology. Novolimus and Sirolimus have been studied as part of Elixir Medical’s Clinical Programs. These clinical trials for DynamX™, DESyne®, DESyne® BD and the fully resorbable DESolve® and ...
Manufactured by:Cingulate based inKansas City, KANSAS (USA)
CTx-1301 utilizes a breakthrough multi-core formulation of dexmethylphenidate, a compound approved by the FDA for the treatment of ...
by:Curemark based inRye Brook, NEW YORK (USA)
The U.S. Centers for Disease Control and Prevention estimate that 1 in 68 children in the United States have been diagnosed with Autism. There is currently no approved drug on the market that treats the core symptoms of Autism. At Curemark, we are working to change that. Curemark’s lead drug candidate, CM-AT, has received “Fast Track” designation from the U.S. Food and Drug ...