- Home
- Equipment
- usa pennsylvania
- in vitro
Refine by
In Vitro Equipment Supplied In Usa Pennsylvania
23 equipment items found
Manufactured by:LifeAire Systems, LLC based inAllentown, PENNSYLVANIA (USA)
Eliminating all known airborne pathogens that can affect healthy embryogenesis, Aire~IVF is clinically proven to significantly improve clinical outcomes. Its patented multi-staged molecular media and UV technologies combine to neutralize up to 99.99% of biological and chemical contaminants within source and return air—in a single pass through the system. No other system, in-room or in-duct, ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Precision Approach to Address the Underlying Cause of Disease: Our second candidate, MuSK-CAART, is designed to treat myasthenia gravis (MG), an autoimmune disease affecting the neuromuscular junction that can lead to motor impairment, muscle weakness, and respiratory ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
A monoclonal globulin is used for the direct fluorescent antibody procedure in the in vitro detection of rabies in the brains and submaxillary glands of animals. This assay from Fujirebio Diagnostics detects all rabies and lyssaviruses tested to date including: street and fixed strains, Duvenhage, Lagos Bat, and Mokola. CE marked. ...
by:Reaction Biology Corp. based inMalvern, PENNSYLVANIA (USA)
Like its counterpart in Drosophila, ASH1L contains a SET histone methyltransferase domain and has been found to play a role in the regulation of Hox gene expression. Although the ASH1L SET domain has been shown in vitro to methylate histone peptides on lysine-4 of histone H3 (H3K4), ASH1L is an H3K36 methyltransferase both in vivo and in vitro with nucleosomes as ...
Manufactured by:Amicus Therapeutics, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Galafold® is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data. This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
New "ray of light" in Vitamin D testing. Lumipulse G 25-OH Vitamin D is an immunoreaction cartridge for in vitro diagnostic (IVD) use with the LUMIPULSE G System for the quantitative determination of 25-hydroxyvitamin D (25-OH-D) in human serum or plasma to be used in the assessment of Vitamin D ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
CanChek Tumor Marker Control Serum is intended for use as an assayed quality control serum to monitor the accuracy of laboratory testing for neuron specific enolase (NSE), S100B protein and squamous cell carcinoma antigen (SCCA). The use of a quality control serum is indicated to verify the performance characteristics of an in vitro diagnostic laboratory test and is an integral ...
Manufactured by:FireGene based inSpringfield, PENNSYLVANIA (USA)
This product is an in vitro diagnostic kit used for the detection of Monkeypox Virus Nucleic Acids. The kit can be used to assess samples from skin swabs, papule or ...
Manufactured by:Invicro, LLC based inNeedham, MASSACHUSETTS (USA)
Invicro has spent 20 years specializing in neuroscience imaging solutions for preclinical, early phase and late phase clinical studies. Our capabilities span from in vitro assays to in vivo imaging using PET, SPECT and MRI imaging biomarkers in CNS drug development and CNS clinical trials. We have expertise in coordinating worldwide multi-center imaging studies, and providing ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. Testing with the OraQuick Ebola Rapid Antigen Test must only be performed when public ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. Testing with the OraQuick Ebola Rapid Antigen Test must only be performed when public ...
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
Introducing a gentle giant in surgical and wound closure. SURGISEAL VET™ and SURGISEAL VET™ PRO Topical Skin Adhesives are fast alternatives to sutures. They offer all the benefits of a traditional skin adhesive and ...
Manufactured by:Shifa Biomedical Corporation based inMalvern, PENNSYLVANIA (USA)
LDLR is a cell-surface receptor that binds LDL-C and carries it into cells to the endosome for degradation and thus reduces the plasma level of LDL-C (Figure 1). However, PCSK9 controls the degradation of the LDLR in the liver (Figure 1) and thereby contributes to cholesterol ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The 45S5 formulation of bioactive glass has been the primary material used in today's bioactive glass bone graft products (45% SiO2, 24.5% CaO, 24.5% Na2O, and 6% P2O5). ...
Manufactured by:Focus Laboratories Inc. based inBethlehem, PENNSYLVANIA (USA)
Once the bioburden of the medical device is understood, appropriate parameters can be established for assuring device sterility. Sterility must be verified, and this is typically accomplished by direct immersion of the devices in media and incubation for times prescribed by USP ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
Effortless, reliable, IVD qPCR system to meet your unique needs. For over 10 years, Thermo Fisher Scientific has developed high-performance, superior, real-time PCR (qPCR) instruments for clinical laboratories all over the world. We have also been committed to developing systems that meet the specific needs of clinical, diagnostic, and assay developers, with the increased security and compliance ...
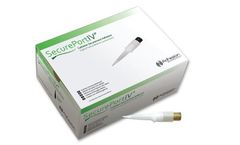
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
The most versatile care and maintenance device in vascular access. Standard of care in vascular access typically involves the use of multiple products for securement and insertion site protection. See how SecurePortIV® Catheter Securement Adhesive is combining multiple patient and caregiver benefits into one ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Our lead product candidate, DSG3-CAART, is being developed to treat mucosal pemphigus vulgaris (mPV), a blistering skin disease that affects mucous membranes. mPV is caused by autoantibodies that attack the cell adhesion protein desmoglein 3, or DSG3. DSG3-CAART is designed to selectively target and kill the B cells that produce DSG3 antibodies while preserving the healthy B cells critical to ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
Create physiologically relevant cell culture models. Gibco Human Plasma-like Medium (HPLM) is a new formulation designed to resemble the natural cellular environment found in the body, mimicking the metabolic profile of human plasma. The widely used classic synthetic cell culture media, including MEM, DMEM, RPMI 1640, and DMEM/F-12 contain glucose, amino acids, vitamins, and salts at ...
Manufactured by:LG Chem, Ltd. based inSeoul, SOUTH KOREA
Intended Use: A reagent for in vitro diagnostic devices that qualitatively detect Clostridium difficile present in human feces or cultured bacteria using PCR test. System: SLAN-96P - Device used to amplify a specific gene (DNA) for diagnosis of ...