Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Coronavirus Disease Equipment Supplied In Usa
11 equipment items found
Manufactured by:Pfizer Inc. based in, NEW YORK (USA)
Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
100 mg for injection VEKLURY is a prescription medicine used to treat adults and children 12 years of age and older and weighing at least 88 pounds (40 kg) with coronavirus disease 2019 (COVID-19) requiring ...
Manufactured by:Genscript based inPiscataway, NEW JERSEY (USA)
SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2), also known as 20SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2), also known as 2019-nCoV, is a positive-sense single-stranded RNA virus. It caused coronavirus disease in 2019 (COVID-19). SARS-CoV-2 contains glycosylated spike (S) protein, which is composed ...
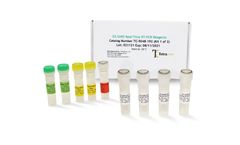
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The Logix Smart™ Coronavirus Disease 2019 (COVID-19) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of the RNA from SARS-CoV-2 coronavirus (COVID-19). The test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The T2SARS-CoV-2 Panel was validated in accordance with the EUA requirements from the FDA and is being distributed in accordance with the FDA guidance on Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency ...
Manufactured by:Tetracore, Inc based inRockville, MARYLAND (USA)
§263a, to perform high complexity tests, or by similarly qualified non-U.S. laboratories. The EZ™-SARS-CoV-2 Real-Time RT-PCR is distributed in accordance with the guidance on Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency, Section ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Tocilizumab (Anti-Human IL6R, Humanized Antibody) can be used for the treatment of rheumatoid arthritis[1]. Tocilizumab is remarkablely effective for the study of severe COVID-19 (coronavirus ...
Manufactured by:Premier Biotech, Inc. based inMinneapolis, MINNESOTA (USA)
The COVID-19 IgG/IgM Rapid Test Cassette is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. COVID-19 (Coronavirus Disease) is the infectious disease caused by the most recently discovered coronavirus. This new virus and ...
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The multiplex test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in lower respiratory tract specimens (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract specimens (e.g. nasopharyngeal, nasal, and oropharyngeal swabs), and saliva from individuals suspected of Influenza A, Influenza B or ...
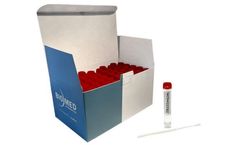
Manufactured by:Biomed Diagnostics, Inc. based inWhite City, OREGON (USA)
Biomed Diagnostics' VTM-C19 Transit Tube is intended for on-site collection and transport to the testing laboratory of human clinical specimens containing SARS-CoV-2 — the virus that causes COVID-19 disease in humans and/or (USA only) influenza A virus. Intended to be inoculated with specimens collected with a nasopharyngeal (NP) or oropharyngeal (OP) synthetic fiber swab (not provided), ...
Manufactured by:KI Canada Ltd. based inPeterborough, ONTARIO (CANADA)
Demron is Certified Against the Coronavirus RST Demron Apparel is Certified Against the Coronavirus Radiation Shield Technologies offers ...