Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Drug Approval Equipment Supplied In Usa
57 equipment items found
by:Starton Therapeutics based inParamus, NEW JERSEY (USA)
STAR-LLD is in development in two continuous delivery systems: subcutaneous and transdermal. STAR-LLD is being developed to establish the only IMiD (immunomodulatory drug) approved for CLL. CLL is the most common form of leukemia. Lenalidomide has been shown to be efficacious and well tolerated in CLL previous randomized controlled trials did not obtain an ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Oral TPOXX® (tecovirimat) is the first drug approved by the U.S. Food and Drug Administration (FDA) that is specifically indicated for the treatment of smallpox disease in adults and pediatric patients weighing at least 13 kg. (Prescription Label) TPOXX® inhibits viral maturation of variola virus (the virus that causes smallpox) and other ...
Manufactured by:Quanterix Corporation based inBillerica, MASSACHUSETTS (USA)
For the first time, oncology and immuno-oncology researchers and others who rely on multiplexing capabilities have an easy-to-use platform to help optimize workflows, speed up their research and ultimately accelerate drug ...
by:Curemark based inRye Brook, NEW YORK (USA)
Current prescription medications and psychosocial therapies focus on ameliorating primary symptoms. No currently approved drug treats the underlying cause of ADHD, and there is no known cure. Our pipeline includes a Phase III-ready drug product for ADHD. Preliminary studies with patients diagnosed with ADHD and taking prescribed ADHD medication ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Dalbavancin (INN, trade name Dalvance) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin.The Food and Drug Administration approved dalbavancin for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible ...
Manufactured by:RareStone Group based inShanghai, CHINA
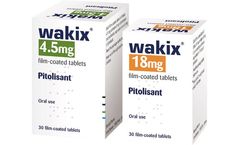
Pitolisant is a selective histamine H3-receptor antagonist/inverse agonist, which increases the release of the brain chemical histamine to increase a patient's wakefulness and alertness. The drug was approved by the European Medicines Agency (EMA) in 2016 for the treatment of narcolepsy in adults with or with0out cataplexy and is marketed in major countries in ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
In October 2018, SIGA entered into a Cooperative Research and Development Agreement (CRADA) with the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) with a focus on Good Laboratory Practice (GLP) studies of TPOXX® for PEP using a U.S. Food and Drug Administration (FDA)-approved non-human primate model of TPOXX® efficacy ...
Manufactured by:Eleven P15 based inDenver, COLORADO (USA)
Idiopathic Pulmonary Fibrosis (IPF) is a chronic, fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and localized to the lung. IPF is a fatal disease with median survival at 3-5 years. Two drugs have been approved for treatment of IPF, but these drugs fail to reverse scarred lung tissue or to ...
Manufactured by:Amivas (US), LLC based inFrederick, MARYLAND (USA)
Artesunate for Injection is an antimalarial indicated for the initial treatment of severe malaria in adults and ...
Manufactured by:EndoLogic LLC based inClifton, NEW JERSEY (USA)
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety ...
Manufactured by:BioQ Pharma based inSan Francisco, CALIFORNIA (USA)
invenious connect-and-go technology is already being used to deliver infusibles in a patented range of infusion systems. Our products are thoughtfully-designed to each drug – with bespoke solutions for: Drugs requiring complex weight-based dosing. Cytotoxic drugs. Drugs that need to be reconstituted or diluted at time of use. Drug-drug combinations, including for cold-chain ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UroGen is developing UGN-102 (mitomycin) for intravesical solution as a primary nonsurgical alternative to repetitive TURBT. UGN-102 is an investigational formulation that utilizes our innovative technology, RTGel™ reverse-thermal hydrogel, for the treatment of low-grade NMIBC. Instilled via standard catheters, UGN-102 is designed to dwell for a period of several hours before being excreted ...
Manufactured by:KORU Medical Systems based inMahwah, NEW JERSEY (USA)
With the performance of the familiar FREEDOM60® in an even simpler design for 20ml and 30ml syringes comes the FreedomEdge® – a compact infusion solution that’s easy to use for smaller or often-repeated doses. It is so simple it makes frequent and flexible dosing a reality – in a safe, controlled manner. Just close and go. ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
GPEx cell line development technology is a proven technology that generates high-performance, highly stable, production cell lines. Virtually any cDNA (mAb and multigenic applications) can be inserted into cells. To date, over 600 different mAb and mAb fusions and over 160+ different recombinant proteins have been produced using the GPEx system, with 160+ clinical trials utilizing therapeutic ...
Manufactured by:Vericel Corporation based inCambridge, MASSACHUSETTS (USA)
The U.S. Food and Drug Administration has approved MACI (autologous cultured chondrocytes on porcine collagen membrane) for the repair of symptomatic, full-thickness cartilage defects of the knee in adult patients. MACI is the first FDA-approved product that applies the process of tissue engineering to grow cells on scaffolds using healthy ...
by:Curemark based inRye Brook, NEW YORK (USA)
Centers for Disease Control and Prevention estimate that 1 in 68 children in the United States have been diagnosed with Autism. There is currently no approved drug on the market that treats the core symptoms of Autism. At Curemark, we are working to change that. Curemark’s lead drug candidate, CM-AT, has received “Fast Track” ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors ...
Manufactured by:Premier Biotech, Inc. based inMinneapolis, MINNESOTA (USA)
Premier Biotech’s CLIA-Waived (Clinical Laboratory Improvement Amendments) multi-drug integrated cup and dip products offer the ability to test for 5-14 drugs. CLIA-Waived test products are cleared by the FDA for over-the-counter (OTC) use, suited for workplace and clinical drug testing programs and approved for waiver ...
Manufactured by:Lipella Pharmaceuticals Inc. based inPittsburgh, PENNSYLVANIA (USA)
Hemorrhagic Cystitis, also called Radiation Cystitis when occurring after pelvic radiation. Lipella is currently conducting a phase-2a, dose-escalation clinical trial evaluating LP-10 (liposomal tacrolimus) for the treatment of hemorrhagic cystitis. Lipella has received US-FDA Orphan Designation for this developmental product. Radiation used to treat prostate, colon, uterine, cervical and other ...