Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Applications
- Microbiome Therapeutics for Patients and Physicians
- Solution for Clinical Trials
- Solution for Access to Investigational Medicines
- Freeze-Dried Hemostatic for Patients
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- RNA Technology for Research Fellowship
- RNA Technology for Medical Areas of Focus Research
- RNA Technology for Product Pipeline Research
- RNA Technology for Clinical trials
Drug Designation Equipment Supplied In Usa
60 equipment items found
by:Vybion based inMonterey, CALIFORNIA (USA)
INT41 has received Orphan Drug Designation from the FDA for Huntington’s disease. IND enabling studies for INT41 Gene Therapy for Huntington’s disease are in place following a preIND meeting with the FDA in in February 2022 in response to the briefing book prepared by our consultants at Latham BioPharm, a world class consulting group based in ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-2900, Tonix’s proprietary potentiated intranasal oxytocin is in the pre-Investigational New Drug (IND) stage as a candidate for the treatment of Prader-Willi syndrome (PWS) and non-organic failure to thrive disease. TNX-2900 has been granted Orphan Drug Designation (ODD) in the US by the FDA for the treatment of ...
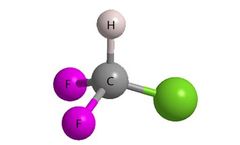
Manufactured by:Alfa Chemistry based inNY, NEW YORK (USA)
Introducing fluorine atom(s) into bioactive organic compounds has become a leading strategy for drug design and lead optimization. In drug synthesis strategy, fluorine is frequently employed to modify biologically relevant properties such as metabolic stability, basicity, lipophilicity and bioavailability. Additionally, the binding affinity of ...
Manufactured by:Cellphire Therapeutics, Inc. based inRockville, MARYLAND (USA)
The product is currently in a Phase 2 clinical trial in bleeding thrombocytopenic patients. Thrombosomes has also been given orphan drug designation for the treatment of Acute Radiation Sickness by the U.S. Food and Drug ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-287 is a consortium of multiple bacterial spores manufactured by fractionating and purifying targeted bacteria from stool of healthy human donors. SER-287 has been granted Fast Track designation and Orphan Drug designation by the FDA. A completed SER-287 Phase 1b clinical study demonstrated a statistically significant difference in the ...
Manufactured by:Protara Therapeutics, Inc. based inNew York, NEW YORK (USA)
Because PN patients cannot sufficiently absorb adequate levels of choline and no available PN formulations contain sufficient amounts of choline to correct this deficiency, PN patients often experience a prolonged progression to hepatic failure and death, with the only known intervention being a dual small bowel / liver transplant. If approved, IV choline chloride would be the first approved ...
Manufactured by:YourChoice Therapeutics based inSan Francisco, CALIFORNIA (USA)
Blocks Cell Division and Blocks Sperm Release enabled by a proprietary ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
Our lead product candidate, DSG3-CAART, is being developed to treat mucosal pemphigus vulgaris (mPV), a blistering skin disease that affects mucous membranes. mPV is caused by autoantibodies that attack the cell adhesion protein desmoglein 3, or DSG3. DSG3-CAART is designed to selectively target and kill the B cells that produce DSG3 antibodies while preserving the healthy B ...
Manufactured by:Notitia Biotechnologies based inMonmouth Junction, NEW JERSEY (USA)
NM108 is an investigational botanical drug that is designed to treat diseases by promoting the growth of either the existing or replenished Foundation Guild™ bacteria in a patient's gut microbiome. NM108 is currently in phase 2 trial as well as available for Expanded Access (compassionate ...
by:DEARGEN Inc. based inDaejeon, SOUTH KOREA
MolEQ designs novel compounds by optimizing multiple properties such as efficacy and toxicity of a lead ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
Dipraglurant is a novel orally available highly selective metabotropic glutamate receptor subtype 5 negative allosteric modulator (mGlu5 NAM) which has completed Phase 1 and demonstrated efficacy in a Phase 2a proof of concept study for the treatment of levodopa-induced dyskinesia associated with Parkinson’s disease (PD-LID). This is a disease with significant commercial opportunity as ...
Manufactured by:Advin Biotech, Inc. based in, CALIFORNIA (USA)
Advin offers a complete line of oral fluid drug tests in a convenient device that may contain up to 12 different drugs. A saturation indicator is included to ensure sufficient specimen collection. The test lineup includes detection of Oxycodone, Tramadol, Ketamine, and Cotinine and many ...
by:Palvella Therapeutics. based inWayne, PENNSYLVANIA (USA)
QTORINTM rapamycin 3.9% is a novel topical therapy being studied for Pachyonychia Congenita (PC). We are currently enrolling our phase 3 study in ...
Manufactured by:Yukon Medical, LLC based inDurham,, NORTH CAROLINA (USA)
The Arisure Dry Spike gives you an easy, safe way to transfer fluid into a bag or container while maintaining a closed ...
Manufactured by:RedHill Biopharma Ltd. based inTel-Aviv, ISRAEL
RHB-204 is a proprietary, fixed-dose oral capsule containing a combination of clarithromycin, rifabutin, and clofazimine, developed for the treatment of pulmonary NTM disease caused by Mycobacterium avium Complex (MAC). ...
Manufactured by:Kala Pharmaceuticals, Inc. based inArlington, MASSACHUSETTS (USA)
AMPPLIFY Drug Delivery Technology is designed to enhance penetration through the mucus barrier and deliver increased concentration of drug to the target ocular tissue. AMPPLIFY Drug Delivery Technology has been shown to improve delivery of loteprednol etabonate compared to traditional suspension eyedrops without ...
Manufactured by:Notitia Biotechnologies based inMonmouth Junction, NEW JERSEY (USA)
BM306 is an investigational live biotherapeutic product that contains the Foundation Guild™ bacteria in a defined consortium. This investigational drug is designed to treat diseases by replenishing the missing or extinct Foundation Guild™ bacteria in a patient's gut ...
Manufactured by:Alfa Scientific Designs, Inc based inPoway, CALIFORNIA (USA)
ALFA’s reliability for speed and accuracy in point of care rapid tests is readily available in the configuration you need. Our Multi-Drug Urine Cassette Test formats vary to our customers’ ...
Manufactured by:Advin Biotech, Inc. based in, CALIFORNIA (USA)
Our new iSplit Cup and POCiT cup are examples of Advin’s focus on innovative engineering that result in improvements in the drug testing field. These designs remove steps in the lab and/or reduce shipping costs and storage footprint without the need for handling sharps or extra components. Both cups have patents pending and are exclusively available from ...
Manufactured by:Montana Molecular based inBozeman, MONTANA (USA)
The Borealis Arrestin Biosensors by Montana Molecular represent a cutting-edge tool in the realm of GPCR drug discovery, focusing on arrestin recruitment and signaling dynamics. These biosensors facilitate the continuous measurement of signaling activities in live cells, crucial for the development of precise pharmacological agents. The sensors are designed to ...