Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Applications
- Solutions for Pharmaceutical
- Microbial Reduction Solutions for Cosmetic and Packaging
- Brain Interfaces Device for Regaining Control
- Brain Interfaces Device for Reconnecting Thought to Action
- Medical Devices for Moderate to Severe Heart Failure Treatment
- Medical Devices for Severe or Advanced Stage Heart Failure Treatment
- Medical Devices for Causes Heart Failure
- Medical Devices for Heart Failure Symptoms
- Medical Devices for Diagnosis and Evaluation
- Heart Pump for Heart Disease
- VenoValve - Surgically Implanted Valve for Chronic Venous Disease (CVD)
- VenoValve - Surgically Implanted Valve for Chronic Venous Insufficiency (CVI)
- Nucleus Replacement System for Degenerative Disc Disease
Implant Device Equipment Supplied In Usa
96 equipment items found
Manufactured by:Healionics based inSeattle, WASHINGTON (USA)
All implanted devices – including prosthetics, ophthalmic, surgical products, sensors, therapeutics delivery, stimulators, percutaneous devices, vascular – share two common problems: The body reacts to the implant and scars the surrounding tissue, causing medical issues, damaging the device and/or ...
Manufactured by:ChoiceSpine LLC based inKnoxville, TENNESSEE (USA)
Hawkeye TI is changing the game for cervical corpectomy cases. With its 510(k) cervical clearing, 8° lordosis, and multiple footprints corpectomy cases are now more efficient than ever. Hawkeye TI is getting surgeons and patients out of the operating room and on the road to recovery faster and more ...
Manufactured by:Impulse Dynamics based inMarlton, NEW JERSEY (USA)
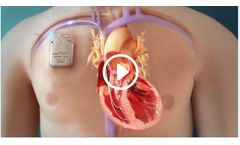
The Optimizer Smart is a minimally invasive implantable device that treats patients experiencing moderate to severe congestive heart failure CHF symptoms even after appropriate medical treatment. The device operates by delivering precisely timed electric pulses called cardiac contractility modulation (CCM) therapy. ...
Manufactured by:STC Material Solutions based inSanta Ana, CALIFORNIA (USA)
Feedthroughs for implantable devices are one of the main products STC Material Solutions supplies. STC Material Solutions played a key role in developing customized feedthroughs for medical implants. All medical feedthroughs are designed and manufactured using only biocompatible materials. ...
Manufactured by:Alafair Biosciences, Inc. based inAustin, TEXAS (USA)
VersaWrap is intended to manage and protect tendon injuries where there is no significant loss of tendon tissue, and is intended to manage peripheral nerve injuries where there is no significant loss of nerve tissue. The device may also be used to manage and protect surrounding tissues such as ligament and skeletal muscle. VersaWrap was designed to separate healing tissue from surrounding tissues ...
Manufactured by:Neuspera Medical, Inc. based inSan Jose, CALIFORNIA (USA)
The Neuspera micro-implant has a modular construction, enabling adaptation for a variety of neural interfaces. The device has an array of programmable stimulation parameters, including frequency, pulse width, and amplitude (voltage) for current control, allowing for maximum flexibility in addressing common neurostimulation applications. Hermetically sealed and ...
Manufactured by:Medicomp Inc. based inMelbourne, FLORIDA (USA)
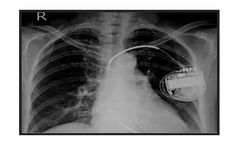
Medicomp's remote monitoring for implantable cardiac devices such as pacemakers, ICDs, and ILRs can help you free up staff time and improve patient ...
Manufactured by:Medtimo Inc based inEden Prairie, MINNESOTA (USA)
The ReShape Vest™ is a revolutionary, anatomy-friendly, laparoscopic, implantable device to enable weight loss and stomach preservation. The procedure restricts stomach volume without cutting, stapling, or removing the ...
by:V-Wave Ltd. based inCaesarea Industrial Park (N), ISRAEL
The V-Wave Ventura Interatrial Shunt is a novel, hourglass-shaped implantable device. The shunt is designed to enable shunting of blood across the interatrial ...
Manufactured by:AK Stamping Company, Inc. based inMountainside, NEW JERSEY (USA)
AK Stamping understands the unique challenges of the medical device market and is equipped to meet them. Our strong engineering and product development capacity, prototyping support, in-house tool build, precision metal stamping capabilities and quality capabilities exceed the demands of our customers, OEM’s and contract manufacturers in the medical market, particularly ...
Manufactured by:Pulmonx Corporation based inRedwood City, CALIFORNIA (USA)
The Zephyr Endobronchial Valve is an implantable device used to occlude all airways feeding the hyperinflated lobe of a lung that is most diseased with ...
Manufactured by:Neuspera Medical, Inc. based inSan Jose, CALIFORNIA (USA)
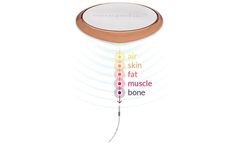
Neuspera’s patented Mid-Field Powering technology uses evanescent and propagating electromagnetic waves to power implanted medical devices to depths of 10cm and beyond. Using the body as a natural waveguide, the Neuspera system can focus power, ensuring energy is delivered to the precise area it is needed. Leveraging efficient power transfer, the power ...
Manufactured by:3DBio Therapeutics based inLong Island City, NEW YORK (USA)
The Overshell is a proprietary technology 3DBio has developed to provide a means for adding non-permanent structural support to biological implants where desirable (e.g. surgical handling and early mechanical function) while supporting their biological function and the patient’s tissue regeneration processes. This is made possible through its unique design ...
Manufactured by:Mustang Vacuum Systems Inc based inSarasota, FLORIDA (USA)
Thin film coatings for medical industry traditional has been focused on surgical tools and cutting tools to ensure sharpness, and longevity . Standard coatings are now available for a wide range of applications to promote adoption by the body for implantable devices such as screws, plates or rods to support repair from traumatic injury as well as the advanced ...
Manufactured by:Silver Bullet Therapeutics, Inc. based inSan Jose, CALIFORNIA (USA)
Medical Device Failures from Device-Related Contaminations are Rampant, Costly and Deadly. What If Implantable Devices Were Antimicrobial? CE-Marked OrthoFuzIon Bone Screw System with a silver ionization coating is an antimicrobial orthopedic implant approved for sale in ...
Manufactured by:BCR Diagnostics Inc based inPhoenix, ARIZONA (USA)
Government guidelines require that a spore test should be used on each sterilizer at least weekly. A spore test should also be used for every load with an implantable device. It has become common practice to test sterilizer daily, but there is a trend to use BIs with each load. In this respect, an inexpensive BI like the BCR-30 min BI affords testing with each ...
Manufactured by:Precision Ceramics USA. based inSt. Petersburg, FLORIDA (USA)
Bioceramics are used in biomedical applications, ranging from medical implants to biomedical pumps. Ceramic materials have been produced for custom practices for centuries, but now they are more of a modern development in medical processes and applications. For decades now there has been a considerable increase in the use of ceramic materials for implant ...
Manufactured by:Tegra Medical based inFranklin, MASSACHUSETTS (USA)
Highlights: Accurate, repeatable holes with excellent surface finishes. Straightness tolerances of .001” (.025mm) per foot. Concentricity tolerances of .001” (.025mm) per inch. Hole diameter tolerances of +/- .0005” (.0127mm). Finish tolerances as low as 4 Ra. Burr-free intersections. Consistent reproduction from hole to ...
by:Theradaptive based inFrederick, MARYLAND (USA)
With our innovative protein-engineering technology, we transform proteins into material-binding ...
Manufactured by:Pulse Technologies based inQuakertown, PENNSYLVANIA (USA)
Electrodes and microelectrode arrays in most long-term implantable neurostimulation devices demand low impedance for sensing and recording applications, and high charge injection capacity to achieve safe and reversible stimulation. Electrodes with larger geometric surface area perform better, but they take up more space, limit the device’s ...