Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
T Cell Receptor Based Cell Therapy Equipment Supplied In Usa
22 equipment items found
Manufactured by:Adaptimmune LLC based inPhiladelphia, PENNSYLVANIA (USA)
Our ADP-A2M4 (MAGE-A4) SPEAR T-cell therapy is directed to a member of the MAGE family of cancer testis antigen expressed in a number of solid tumor cell types. The MAGE- A4 antigen is among the most commonly expressed cancer-testis antigens. We are conducting various trials with the ADP-A2M4 SPEAR T-cell in various solid ...
Manufactured by:Adaptimmune LLC based inPhiladelphia, PENNSYLVANIA (USA)
Our ADP-A2M10 (MAGE-A10) T-cell therapy is directed to a member of the MAGE family of cancer testis antigens expressed in a number of solid tumor cell types. Any expression in cancer is often associated with higher grade tumors. Please note that the two trials with ADP-A2M10 are now closed for ...
Manufactured by:Adaptimmune LLC based inPhiladelphia, PENNSYLVANIA (USA)
Our ADP-A2AFP SPEAR T-cell product targets alpha-fetoprotein (AFP) is being investigated in an ongoing Phase 1 clinical trial for the treatment of patients with hepatocellular carcinoma (liver ...
Manufactured by:IN8Bio Inc. based inNew York, NEW YORK (USA)
INB-200, our DeltEx DRI, is a genetically modified autologous gamma-delta T cell product candidate for the treatment of solid tumors. This is the first genetically engineered gamma-delta T cell therapy to be administered to patients and our initial indication is newly diagnosed Glioblastoma (GBM). Since 2005 the standard of care treatment for GBM has been surgical resection followed by radiation ...
Manufactured by:Trailhead Biosystems Inc. based inBeachwood, OHIO (USA)
We are developing cell culture media for T-cell subtype control to improve clinical potency in the individual patient and reduction of variability in outcomes between patients. CAR T-cell therapy involves the treatment of cancer by modifying a patient’s T-cells in the lab so they will attack cancer cells. If clinical development of the CART T-cell produces the wrong T-cell subtype, patient ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
Licensed from the National Institutes of Health, VCAR33 is a CD33-directed chimeric antigen receptor T cell (CAR-T) therapy. A T cell therapy using the same CAR construct as VCAR33 is being studied in a multi-site Phase 1/2 clinical trial as an autologous monotherapy bridge-to-transplant for relapsed and/or refractory AML patients, sponsored by the National Marrow Donor Program ...
Manufactured by:T-Cure BioScience, Inc. based inSherman Oaks, CALIFORNIA (USA)
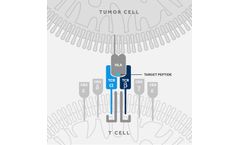
T cell engineering with TCRs is a growing area of immuno-oncology with recent breakthroughs in clinical settings. The T-Cure approach involves identifying and isolating a high avidity TCR which specifically recognizes an intracellular or cell-surface target in solid tumors. This TCR is then typically introduced into a viral vector that can deliver the TCR into the patients’ own T cells in ...
Manufactured by:Adaptimmune LLC based inPhiladelphia, PENNSYLVANIA (USA)
Our ADP-A2M4CD8 SPEAR T-cell therapy is our first "next-generation" therapy and is directed to MAGE-A4, a member of the MAGE family of cancer-testis antigens expressed in a number of solid tumor types. What differentiates this therapy from our ADP-A2M4 SPEAR T-cells is that these cells also express the CD8α co-receptor alongside the engineered TCR that targets MAGE-A4. Preclinical data ...
Manufactured by:Adaptive Biotechnologies based inSeattle, WASHINGTON (USA)
We are currently leveraging our TCR discovery capabilities to enable commercialization of novel therapies by collaborators. In the future, we may explore expanding our end-to-end capabilities for the development of cellular therapies and vaccines. TruTCR is summarized in the figure ...
by:Caribou Biosciences, Inc. based inBerkeley, CALIFORNIA (USA)
CAR-T cell therapies are a class of treatments in which T cells are modified to express chimeric antigen receptors (CARs). CARs are engineered molecules that, when present on the surface of a T cell, enable a T cell to recognize a specific protein that is present on the surface of other cells, including cancer cells. Upon recognition of the target cell via the CAR, the CAR-T cell kills the ...
Manufactured by:Aptevo Therapeutics based inSeattle, WASHINGTON (USA)
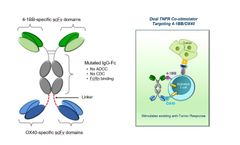
APVO603 is a dual agonist bispecific antibody employing a novel mechanism of action to simultaneously target 4-1BB (CD137) and OX40 (CD134), both members of the TNF-receptor family. Dual targeting of 4-1BB and OX40 provides synergistic co-stimulation of T cells with the potential to amplify the cytotoxic function of activated T cells and NK cells, potentially leading to more robust anti-tumor ...
Manufactured by:Tevogen Bio based in, NEW JERSEY (USA)
Innovative Science in Virology, Oncology, and Neurology; Allogeneic CD8+ T cell therapies from a single donor to treat thousands for the first ...
Manufactured by:Celdara Medical, LLC based inLebanon, NEW HAMPSHIRE (USA)
Celdara Medical gives hope and health to patients by transforming academic innovations into medicines that cure the world’s most challenging ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Probody therapeutics are designed to outsmart cancer by exploiting the unique conditions of the tumor microenvironment to more effectively localize treatment to the tumor, while limiting activity in healthy tissues. These novel therapies are designed to take advantage of high levels of protease activity in the tumor microenvironment. Their target-binding regions are masked to limit activity ...
Manufactured by:Ardigen based inKraków, POLAND
Quick solution for effective immune responses in clinical trials; We have created an Artificial Intelligence platform dedicated to being a part of the therapeutic cancer vaccine development. The ArdImmune Vax platform uses sequencing data to generate and prioritize antigen targets for cancer vaccines and T-cell ...
Manufactured by:Immatics N.V. based inTuebingen, GERMANY
Bispecific T cell engaging receptors (TCER®) are off-the-shelf biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing specific tumor targets. The design of these novel biologics allows any T cell in the body to become activated and attack the tumor, regardless of the T cells’ intrinsic ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
CAR T-cell therapy has recently emerged as a revolutionary and potentially curative therapy for patients with certain hematologic malignancies, including refractory cancers. In 2017, two CAR T-cell therapies were approved by the FDA for the treatment of relapsed / refractory B-cell precursor acute lymphoblastic leukemia (ALL) and relapsed / refractory diffuse large B-cell lymphoma (DLBCL). While ...
Manufactured by:Access Biologicals LLC based inVista, CALIFORNIA (USA)
Human AB Serum lacks antibodies against the A and B blood type antigens, which prevents reaction with cultured cells and is recommended for use in biomedical applications. Human AB serum is regularly used for tissue engineering, transplantation and expansion of T-cells, cell therapy applications and cell line testing for PBMCs, bone marrow, CD9, CD3 and CD33. In addition, Human Male AB Serum is ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
T cells engineered with chimeric antigen receptors (CARs) have shown exceptional promise as a potentially curative therapy for patients with certain hematologic malignancies. While most researchers and clinical investigators continue to focus on the development of autologous or allogeneic CAR T-cell therapies, we are developing CAR NK cell product candidates created from clonal master engineered ...
by:Intellia Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
We plan to initiate patient screening for a first-in-human Phase 1 study of NTLA-5001, a WT1-directed TCR T cell therapy for the treatment of AML, by the end of 2021. Our first ex vivo development candidate seeks to treat acute myeloid leukemia (AML) by engineering autologous T cell receptors (TCR) directed towards the Wilms’ Tumor 1 (WT1) antigen, an over-expressed protein that is often ...