Refine by
Clinical Regulations Software Available In Croatia
10 software items found
by:Clinion based inHyderabad, INDIA
Using AI to Automate Study & Submission Documentation. Automate Protocol And Clinical Study Reports With The Help Of Machine ...
by:SK-Telemed GmbH based inVienna, AUSTRIA
Creation of EHR- information about the patient from the appointment to the discharge from the hospital is preserved and available to all participants in the treatment process Possibility of integrating EHR with other information systems and registries for obtaining additional information about the patient Intergated- classifiers of medicines, medical services and clinical protocols The ...
by:AWS Truepower, LLC based inAlbany, NEW YORK (USA)
Discover how our validated, FDA CFR Part 11 compliance training management system helps you comply with evolving FDA Regulations. Keep your workforce up to date. Medical device and pharmaceutical manufacturers trying to keep up with ever-changing regulations have a tough job. Medical technology advances quickly, and with it the need for compliance systems to ...
by:Jeeva Informatics Solutions Inc. based inManassas, VIRGINIA (USA)
Clinical research sites, sponsors, and CROs, minimize the logistical burdens of paper based process for gathering patient reported outcomes, patient diaries, patient satisfaction, and quality of life questionnaires. Whether standardized or custom on a per-clinical-trial subscription or enterprise license. The modular subscription service is based on number of patients and can scale as the study ...
by:Jeeva Informatics Solutions Inc. based inManassas, VIRGINIA (USA)
Clinical research sites, sponsors, and CROs, minimize the logistical burdens of the informed consent process on a per-clinical-trial subscription or enterprise license. The modular subscription service is based on number of patients and can scale with the study enrollment process. Automate the manual repetitive tasks of explaining the study protocol, risks, benefits, samples or data use, and ...
by:Medidata based inNew York, NEW YORK (USA)
Medidata AI Integrated Evidence connects historical data from more than 23,000 cross-sponsor clinical trials and 7 million patient volunteers with real world data to deliver powerful insights and the necessary evidence clinical development leaders need to increase the probability of trial success. Artificial intelligence and data can help fuel new ways of working on clinical trials and beyond. ...
by:CureMetrix, Inc. based inSan Diego, CALIFORNIA (USA)
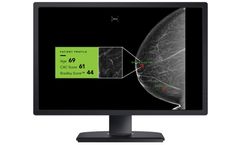
cmTriage™ from CureMetrix is the first FDA-cleared software in the U.S. intended to provide a notification triage code to the radiologist’s mammography worklist based on the presence of a suspicious region of interest found by the underlying algorithm. This workflow optimization tool enables a radiologist to customize their 2D mammogram or mammography worklist based on cases that may ...
by:MVision AI based inHelsinki, FINLAND
Utilizing models adhering to international best practices, Contour+ assures standardized contours which enhance inter-clinic collaboration. It significantly decreases manual effort and contour variability, offering up to a 95% reduction in workload. The software integrates seamlessly with existing treatment planning systems and caters to stringent data protection standards such ...
by:CureMetrix, Inc. based inSan Diego, CALIFORNIA (USA)
cmAngio An investigational AI-based triage software that helps doctors assess a patient’s risk of coronary heart disease ...
by:PathAI, Inc. based inBoston, MASSACHUSETTS (USA)
AISightTM is a web-based digital pathology slide viewing platform that enables the management and analysis of whole slide ...