- Home
- Software
- asia middle east
- clinical screening trial
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- Hong Kong
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Clinical Screening Trial Software In Asia Middle East
27 software items found
Manufactured by:Quantificare Inc. based inSuwanee, GEORGIA (US) (USA)
Forget time-intensive imaging software. Keep your investigators happy and enhance their imaging workflow with a comprehensive web solution. DermaPix® Web Portal is the end-to-end solution for the upload, management, monitoring and real-time review of digital images in multicenter clinical ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeBUILDER® provides an intuitive interface for sponsors, CROs, and academic institutions to access and rapidly build cubeCDMS®, cubeIWRS®, and cubePRO® system environments to conduct clinical trials electronically and securely on the cloud. cubeBUILDER® is free, easy-to-learn, and ...
by:Clinion based inHyderabad, INDIA
Clinion's Electronic Trial Master File (eTMF) software optimizes clinical trial management with secure, efficient, and compliant document ...
by:EvidNet Inc. based inSeongnam-si, SOUTH KOREA
Through EVIX-FIND™, EvidNet provides fast-track aggregated patient count query services for your eligibility criteria of interest. ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
In the paperless era, the clinical trial industry is changing. Until now, the Trial Master File (TMF) has consisted of traditional paper documents, images, or media organized in a binder stored in file cabinets. In accordance with FDA's CFR 21 Part 11, we have developed cubeTMF to provide a validated system for a cohesive workflow for a truly paperless clinical trial. cubeTMF® streamlines the ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
Simplify your data life cycle to enable alignment and unlock efficiency across your clinical ...
by:Clinion based inHyderabad, INDIA
Our Electronic Data Capture Software Making Clinical Trials Faster and Better. Embrace Integration: Integrate Clinion Electronic Data Capture Software with Clinion CTMS, IWRS/RTSM, and ePRO/eCOA for true end-to-end support of your Study, obviating the hassles of data reconciliation while dealing with multiple eClinical ...
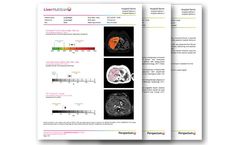
Manufactured by:Perspectum based inOxford, UNITED KINGDOM
Get the whole picture of liver health with LiverMultiScan - a suite of metrics designed to meet the growing demand for liver diagnostics. LiverMultiScan gives physicians key indications of liver tissue characteristics to empower their diagnostic and patient management ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeRBM® is a real-time monitoring solution that helps mitigate risk by planning and ensuring the quality of clinical trials by identifying, evaluating, monitoring and alleviating the risks that may affect the quality and safety of clinical trials. cubeRBM® collects the risks of each site participating in the clinical study in real-time through the EDC and evaluates and compares it with a ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeCTMS® is a clinical trial management system for real-time central monitoring allowing users to view and manage information on the progress of a clinical trial and milestone at a glance. Additionally, cubeCTMS® provides users with effective resource allocation and risk-based monitoring can be planned, taking into account risk areas identified as issue tracking. Conduct clinical trials ...
Manufactured by:Perspectum based inOxford, UNITED KINGDOM
Atlas is a metabolic software suite redefining multi-organ assessment for clinical trials in diabetes and metabolic ...
Manufactured by:Intelligence based inEschborn, GERMANY
Innoplexus recognizes the unique requirements of the life sciences industry and offers custom solutions on top of our products to enable pharmaceutical and biotech companies as well as CROs to have timely and easy access to useful insights. Our proprietary AI technology combined with a self-learning life sciences ontology that understands the context of the search query and our capability to ...
by:Clinion based inHyderabad, INDIA
Decentralise Your Trials And Improve Patient Engagement And Adherence. Decentralise Your Trials With Clinion eCOA For Enhanced Real-World ...
Manufactured by:Perspectum based inOxford, UNITED KINGDOM
This ground-breaking new quantitative imaging tool uses computational techniques to enhance MRCP images. MRCP+ combines true 3D rendering of the enhanced data with quantitative characterization, facilitating clear mapping of the biliary tree and pancreatic ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
Building your IRT system on PULSE allows you to take back control of complex processes. The reporting and messaging functionality helps stakeholders perform day-to-day tasks more efficiently and ...
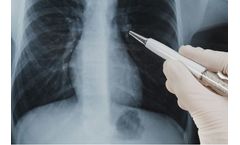
by:Vuno Inc. based inSeocho-gu, SOUTH KOREA
VUNO Med- Chest X-ray has been trained on major abnormal findings detected in chest X-ray to assist in the reading of chest X-ray ...
by:Clinion based inHyderabad, INDIA
Integrated Solution To Plan, Manage and Track All Your Studies. An Integrated Trial Management System That Gives CROs And Sponsors Greater Control And Visibility Into Their Clinical ...
Manufactured by:Intelligence based inEschborn, GERMANY
The clinical trial prediction (CTP) engine empowers your decision-making: Predict the probability of clinical trial success, Evaluate-investment-decisions, Track clinical trial KPIs and identify critical path. Leverage AI to predict clinical trial outcomes: There are a lot of statistics about drug development – and all of them show that the process is inefficient and expensive: Pharma ...
by:Endpoint Clinical based inWakefield, MASSACHUSETTS (USA)
DRIVE: Clinical Supply Management Solution. An enterprise-level solution, DRIVE, enables you to manage clinical supplies at the sponsor, depot, and site levels, providing your team with full visibility and traceability of inventory across all trials, inclusive or exclusive of the use of an ...
by:Insilico Medicine based inPak Shek Kok, HONG KONG
A roadmap to successful disease research and target identification strategies requires considerable resources spent on search, collection and interpretation of data during several months of work. PandaOmics provides a unique opportunity to both explore the unknown of OMICs data and interpret it in the context of all the scientific data generated by the scientific ...