Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Pharmaceutics Software In Usa
50 software items found
by:Chemstations Inc. based inHouston, TEXAS (USA)
Pharmaceutical Excipients is an essential resource for formulators in the pharmaceutical industry, as well as those interested in the formulation or production of confectionery, cosmetics, and food products. It provides scientists with a guide to the uses, safety, chemical and physical properties of pharmaceutical ...
by:RegScan - by Enhesa based inWilliamsport, PENNSYLVANIA (USA)
The starting point are the national regulations of the United States and the European ...
Manufactured by:Particle Measuring Systems (PMS) based inBoulder, COLORADO (USA)
The top Data Collection and leading Data Management companies combined to create a complete contamination monitoring solution for pharmaceutical manufacturers. Plus, with its unique plug and play ability, portable devices and monitoring, system connection has never been ...
by:ToxPlanet by Enhesa based inArlington, VIRGINIA (USA)
ToxPlanet streamlines the process of finding and accessing critical drug-related information with DrugEXPERT. DrugEXPERT delivers access to detailed and up-to-date health and safety data on thousands of pharmaceutical ...
by:Intelligen based inScotch Plains, NEW JERSEY (USA)
SuperPro Designer facilitates modeling, evaluation and optimization of integrated batch and continuous processes in a wide range of industries (Biotech, Pharmaceutical, Specialty Chemical, Food Processing, Consumer Goods, Metallurgical, Materials, Water Purification, Wastewater Treatment, Air Pollution Control, etc.). The combination of manufacturing and environmental ...
by:TriNetX, LLC based inCambridge, MASSACHUSETTS (USA)
Trials that include patients in Japan have an advantage when it comes to regulatory approval in that country, where pharmaceutical sales reached 94B USD in 2020. But approval requires promising results from a well-executed study. Start the process the right way by understanding Japan's patient population, designing a feasible study, and selecting the right trial sites. ...
Manufactured by:Vimachem based inAstoria, NEW YORK (USA)
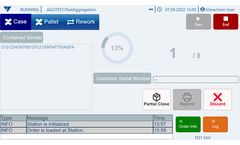
The Vimachem Pharma MES Platform offers an advanced Manual Aggregation Module specifically tailored for pharmaceutical applications. This software solution is a crucial component of Vimachem’s open serialization platform, facilitating seamless connectivity with various IT systems and third-party equipment. Designed for manual, low-speed lines, the module supports the manual ...
Manufactured by:Teledyne Hanson Research based inChatsworth, CALIFORNIA (USA)
Fast, Easy Drag-and-Drop Protocol Management for Vision® and CD testers. Teledyne LABS helps ensure the world’s pharmaceuticals are pure, safe, and effective by manufacturing instruments that set the global standard for quality, innovation, and long-term value. Software and software management play a large role in making sure those systems run as they should, and ...
by:Certara based inPrinceton, NEW JERSEY (USA)
This comprehensive package includes integrated data preparation, modeling, and graphics tools with the same user interface that is used in Phoenix WinNonlin. 18 of the top 20 pharmaceutical companies in the world use Phoenix NLME. ...
by:FreeThink Technologies Inc. based inMontvale, NEW JERSEY (USA)
The ASAPprime software is the only commercially-available software that enables pharmaceutical scientists to quickly and accurately determine drug substance and drug product shelf-life based on the Accelerated Stability Assessment Program (ASAP). This state-of-the-art, statistical software allows prediction of small-molecule drug product use periods, shelf-lives and drug ...
Manufactured by:Intelligence based inEschborn, GERMANY
Transform, clean, and aggregate under-utilized enterprise data in ready-to-analyze state to generate visual insights. Generate real-time information and overview of all functions of a pharmaceutical company, including product performance, sales performance, budget and expenses, patient activity, call center performance, CI landscape, etc. It applies proprietary AI technologies to ...
by:Körber Pharma GmbH based inLüneburg, GERMANY
Right First Time", "Review by Exception", "Paperless Production", "Electronic Batch Recording", "Faster Time to Market", "FDA Compliance" – these are only some of the benefits of using Werum's out-of-the-box MES software product PAS-X for your pharmaceutical and biopharmaceutical ...
by:Simulations Plus based inLancaster, CALIFORNIA (USA)
Modules like PKanalix® support compartmental and non-compartmental analyses, while Simulx® excels in clinical trial simulations, enhancing decision-making in drug development. MonolixSuite aims to streamline complex analyses, making it a valuable asset for pharmaceutical researchers and ...
by:Cytel based inWaltham, MASSACHUSETTS (USA)
As the character of the pharmaceutical industry changes, the need to create innovative clinical trials that de-risk uncertainty and optimize statistical design brings several advantages. The rise in the number of such adaptations also increases the ability for statistical design to be tailored to the needs of a specific trial, thereby keeping patients safe and increasing the ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
OOS Software to Automate Out of Specification (OOS) Processes to Comply with FDA CGMPs and 21 CFR Part 211 and Part 820 Regulations. In the FDA environment, specifications are essential in maintaining quality. CGMP regulations for finished pharmaceuticals (21 CFR Parts 210-211) and medical devices (21 CFR Part 820) require strict conformance to approved specifications which can ...
by:Indica Labs Inc. based inAlbuquerque, NEW MEXICO (USA)
With unmatched ease-of-use and scalability, powerful analytic capabilities, and the fastest processing speeds available for digital pathology, pharmaceutical, healthcare and research organizations worldwide are using HALO for high-throughput, quantitative tissue analysis in oncology, neuroscience, metabolism, toxicology and more. ...
by:Strateos Inc. based inMenlo Park, CALIFORNIA (USA)
Through a combination of best-in-class robotics and control systems, advanced software for imaging and analytics, and remote cloud laboratories, we enable our pharmaceutical partners to more rapidly and efficiently discover new drug ...
by:Certara based inPrinceton, NEW JERSEY (USA)
It is the industry standard for non-compartmental analysis (NCA), pharmacokinetic/pharmacodynamic (PK/PD), and toxicokinetic (TK) modeling with a proven 30-year history. Regulatory agencies, including the US FDA, Japan Pharmaceutical and Medical Device Agency (PMDA), China Food and Drug Administration (CFDA), and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), ...
Manufactured by:Intelligence based inEschborn, GERMANY
Innoplexus recognizes the unique requirements of the life sciences industry and offers custom solutions on top of our products to enable pharmaceutical and biotech companies as well as CROs to have timely and easy access to useful insights. Our proprietary AI technology combined with a self-learning life sciences ontology that understands the context of the search query and our ...
by:Molsoft LLC based inSan Diego, CALIFORNIA (USA)
Each data element can be linked to one another and annotated and then shared via the web, MS Office tools or in the free ICM-Browser. Pharmaceutical and biotech companies use ActiveICM to share data between scientists in different disciplines. Academic laboratories such as the SGC publish their data to the world using ActiveICM in a web ...