Show results for
Refine by
Approved Drug Equipment & Supplies In Monaco
48 equipment items found
Manufactured by:Cellworks Research India Private Limited based inBangalore, INDIA
The Cellworks Platform biosimulates how a patient’s unique cancer will respond to therapies prior to treatment and identifies novel drug combinations for treatment-refractory patients. Therapy biosimulation is the ability to predict the phenotype response of a human cell to an external stimulus, such as a drug ligand or radiation. We utilize this ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Many intravenously delivered Active Pharmaceutical Ingredients (APIs) are insoluble or poorly soluble in water. This can be a major hurdle in pharmaceutical development and may cause promising drugs to fail during the development process and may limit the application of approved drugs because of poor solubility. According to some estimates, ...
Manufactured by:Cerevast Medical, Inc. based inBothell, WASHINGTON (USA)
The formation or deposition of these clots in the arterial vessels of the brain is one of the primary causes of ischemic stroke. Intravenous thrombolytic therapy (tPA/alteplase, the only approved drug for the treatment of ischemic stroke) works by converting plasminogen in the blood stream to plasmin, the active enzyme that dissolves ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors ...
by:Sibylla Biotech S.p.A. based inVerona, ITALY
In Silico: Identifying small molecules able to revolutionize drug discovery. The PPI-FIT technology allows the exploration of unique pharmacological targets represented by protein folding intermediates. This places Sibylla Biotech in the exclusive position of identifying hit/lead compounds with a mechanism of action never explored so far. ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Mobilized Leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate the migration of CD34+ hematopoietic stem and progenitor cells (HSPCs) from the bone marrow niche into the circulation. Mobilized peripheral blood, enriched in single-donor CD34+ HSPCs is collected through leukapheresis using the Spectra ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
N,N-Dimethylacetamide (DMAc) is an organic solvent with blood-brain transmissibility and an FDA-approved drug excipient. N, N-dimethylacetamide exerts anti-inflammatory activity by inhibiting the NF-κB signaling pathway. N, N-dimethylacetamide can be used in studies of weight gain caused by a high-fat diet and neuroinflammation in Alzheimer's ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
The emerging global threat from COVID-19 demanded immediate action. With cases rising rapidly, we set up a specialist team to use our AI-drug discovery platform to search for existing drugs — already proven safe — that could be repurposed to treat the virus. We are the only AI company to successfully identify a drug to treat COVID-19. ...
Manufactured by:BioMark Diagnostics Inc. based inRichmond, BRITISH COLUMBIA (CANADA)
BioMark’s initial liquid biopsy assay detects SSAT1 using an FDA approved drug. SSAT1 is an enzyme with elevated levels in numerous cancers. The assay targets a well researched and understood metabolic pathway. BioMark of a few unique companies using safe therapeutic agents for diagnostic indication. As a non-invasive cost effective red alert tool, it is ...
Manufactured by:Beldimed s.a. based inAndrimont, BELGIUM
The expression “Patient First” is part of our DNA. We truly believe that every person in the world should have the same opportunities when it comes to healthcare because health is the ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Dalbavancin (INN, trade name Dalvance) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin.The Food and Drug Administration approved dalbavancin for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible ...
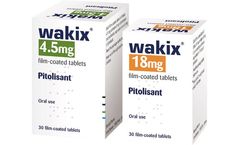
Manufactured by:RareStone Group based inShanghai, CHINA
Pitolisant is a selective histamine H3-receptor antagonist/inverse agonist, which increases the release of the brain chemical histamine to increase a patient's wakefulness and alertness. The drug was approved by the European Medicines Agency (EMA) in 2016 for the treatment of narcolepsy in adults with or with0out cataplexy and is marketed in major countries in ...
Manufactured by:Amivas (US), LLC based inFrederick, MARYLAND (USA)
Artesunate for Injection is an antimalarial indicated for the initial treatment of severe malaria in adults and ...
by:Genmab A/S based inCopenhagen V, DENMARK
Daratumumab is a human IgG1k monoclonal antibody (mAb) that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells.1 Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license to develop, manufacture and commercialize daratumumab from Genmab. A subcutaneous formulation of daratumumab, DARZALEX ...
Manufactured by:BioQ Pharma based inSan Francisco, CALIFORNIA (USA)
invenious connect-and-go technology is already being used to deliver infusibles in a patented range of infusion systems. Our products are thoughtfully-designed to each drug – with bespoke solutions for: Drugs requiring complex weight-based dosing. Cytotoxic drugs. Drugs that need to be reconstituted or diluted at time of use. Drug-drug combinations, including for cold-chain ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:Quanterix Corporation based inBillerica, MASSACHUSETTS (USA)
For the first time, oncology and immuno-oncology researchers and others who rely on multiplexing capabilities have an easy-to-use platform to help optimize workflows, speed up their research and ultimately accelerate drug ...
Manufactured by:KORU Medical Systems based inMahwah, NEW JERSEY (USA)
With the performance of the familiar FREEDOM60® in an even simpler design for 20ml and 30ml syringes comes the FreedomEdge® – a compact infusion solution that’s easy to use for smaller or often-repeated doses. It is so simple it makes frequent and flexible dosing a reality – in a safe, controlled manner. Just close and go. ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
GPEx cell line development technology is a proven technology that generates high-performance, highly stable, production cell lines. Virtually any cDNA (mAb and multigenic applications) can be inserted into cells. To date, over 600 different mAb and mAb fusions and over 160+ different recombinant proteins have been produced using the GPEx system, with 160+ clinical trials utilizing therapeutic ...
by:Pureims B.V. based inRoden, NETHERLANDS
Amikacin Cyclops™ is an amikacin dry powder inhaler for the treatment of tuberculosis (TB). Inhaled amikacin from the Cyclops™ will result in higher amikacin concentrations in the lungs than conventional infusion or intramuscular injection. This could more rapidly decrease contagiousness by sterilizing the upper airways of patients with ’open’ (smear positive) TB, ...