Refine by
Human Immunodeficiency Virus Equipment & Supplies
47 equipment items found
Manufactured by:Jericho Sciences, LLC based inDurham (RTP), NORTH CAROLINA (USA)
HIV-1 is a virus that attacks the immune system in humans. It targets CD4+ T cells (a type of immune cell) that are crucial for fighting off infections. If left untreated, the virus can impair the immune system’s ability to fight off other infections and diseases. ...
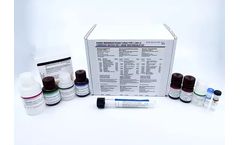
Manufactured by:InTec PRODUCTS, INC. based inXiamen, CHINA
The Advanced HIV Ag-Ab ELISA Test is an ELISA test intended for the qualitative and di?erential detection of antibodies to human immunode?ciency viruses types 1 and 2 (HIV-1 and HIV-2) and simultaneous detection of HIV-1 p24 antigen in human serum or plasma. It is the 4th generation of ELISA HIV test kit which is intended for the screening of blood donors and for aid in the diagnosis of clinical ...
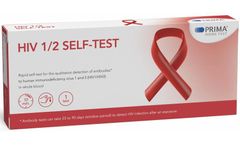
Manufactured by:Prima Lab SA based inBalerna, SWITZERLAND
Rapid self-test for the qualitative detection of antibodies against human immunodeficiency virus 1 and 2 (HIV1/HIV2) in whole blood. Human immunodeficiency virus, or HIV, is a pathogen that attacks and suppresses the immune system by specifically affecting white blood cells. The ...
by:Merck & Co., Inc., based inRahway, NEW JERSEY (USA)
CRIXIVAN (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 200 and 400 mg of indinavir (corresponding to 250 and 500 mg indinavir sulfate, respectively). ...
by:Osel Inc. based inMountain View, CALIFORNIA (USA)
The natural microbiota at these sites, may neutralize some viral particles before infection of the host can occur. Using a proprietary technology, MucoCept harnesses the human microbiome and enhances it to further virus neutralization. In the current MucoCept platform technology, we have modified a commensal Lactobacillus to express a potent inhibitor of ...
by:Evofem Biosciences, Inc. based inSan Diego, CALIFORNIA (USA)
We are collaborating with Orion Biotechnology to evaluate the compatibility and stability of Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product candidate for indications including the prevention of human immunodeficiency virus (HIV) in women. This collaboration ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSDx HIV 1/2 Test is an immunoassay used for the qualitative detection of antibodies to human immunodeficiency virus in whole ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
MaximBio's Cambridge Biotech HIV-1 Urine Western Blot Kit is an FDA approved in-vitro qualitative assay for the detection and identification of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) contained in human urine. This more specific assay is used as a supplemental test with urine specimens that tested repeatedly ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Selgantolimod (GS-9688) is an orally active, potent and selective toll-like receptor 8 (TLR8) agonist for the treatment of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 status can be made, specimens that ...
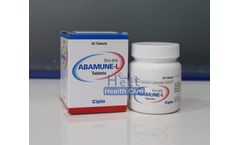
Manufactured by:Heet Healthcare Pvt. Ltd. based inAhmedabad, INDIA
Abamune-L tablets 600mg/300mg is a combination of two medications, abacavir and lamivudine which belong to a class of medicines called antiretroviral. They are used together with other antiretroviral to slow down the progression of HIV infection (human immunodeficiency virus), which can lead to AIDS (Acquired Immune Deficiency Syndrome) and other ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Hypocrellin A is also a photosensitizer for photodynamic therapy (PDT) with anticancer, antibacterial and antiviral activities, especially against human immunodeficiency virus (HIV). In addition, Hypocrellin-A also possesses anti-Leishmania activity (IC50=0.27 ...
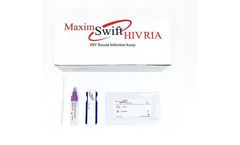
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim Swift HIV Recent Infection Assay (RIA) is a single use qualitative immunoassay to detect the circulating antibodies to Human Immunodeficiency Virus Type 1 (HIV-1), Type 2 (HIV-2) and distinguish between recent and long-term infection in HIV-1/2. The assay is a point of care (POC) test intended for use with blood or serum/ plasma ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Dextran sulfate sodium salt (MW 35000-45000) may be related to macrophage dysfunction, intestinal flora dysbiosis, and is particularly toxic to the colonic epithelium. Dextran sulfate sodium salt (MW 35000-45000) also inhibits human immunodeficiency virus replication by preventing viral adsorption to host cells. Dextran sulfate sodium salt (MW ...
Manufactured by:Impladent Ltd. based inJamaica, NEW YORK (USA)
This product contains Human Allograft Material (reffered to as "graft") collected from human cadaveric donors. A donor serum sample is tested by a CLIA Certified Lab using FDA licensed screening tests and found to be negative or non-reactive for antibodies to human immunodeficiency virus type 1 and type 2 ...
Manufactured by:Retractable Technologies, Inc. (RTI) based inLittle Elm, TEXAS (USA)
Prepare and give injection using aseptic technique according to institutional policy. For injection into patients, continue depressing plunger to activate automatic needle retraction while needle is still inpatient Full dose is administered only when needle retraction is ...
Manufactured by:Airx Laboratories based inFolcroft, PENNSYLVANIA (USA)
RX 15 has always been germicidally effective against the Gram positive and Gram negative test organisms required to be killed for registration by the EPA as a disinfectant cleaner, but it has not been recommended for use in hospitals because it did not kill the hospital critical germ Pseudomonas aeruginosa. ...
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
NeoGuard is a rolled cover, made with material specially designed for ultrasound use. Recommended for patients and professionals with latex protein sensitivities, these high-quality covers are available for popular endocavity transducers. Covers include colored elastic bands. Sterile gel packets available separately. Not made with natural rubber ...
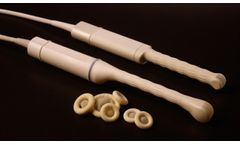
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
Introducing the Intuit line of transducer covers utilizing CIVCO's premium CIV-Flex™ material and delivering simplified application mechanisms for an improved user experience across all sterile ultrasound procedures. The Intuit series allows convenient placement of gel and simple application onto the transducer along with a three-dimensional “box end” for distortion-free ...
Manufactured by:CIVCO Medical Solutions based inCoralville, IOWA (USA)
Especially popular for OB/GYN scanning procedures, CIV-Flex is a soft, pliable material comfortable for patients. The durable CIV-Flex material is acoustically transparent, offering distortion-free imaging. Designed specifically for ultrasound use, CIV-Flex slides easily over transducers and is a recommended alternative for latex-sensitive patients and professionals. Sterile cover kits include ...