- Home
- Equipment
- usa california
- stem cell ideas into effective therapies
Show results for
Refine by
Stem Cell Ideas Into Effective Therapies Equipment & Supplies In Usa California
22 equipment items found
Manufactured by:Kangstem Biotech Co., Ltd. based inGangnam-gu, SOUTH KOREA
FURESTEM-AD Inj. is the world’s first stem cell therapeutic product for atopic dermatitis that targets moderate to severe atopic dermatitis ...
by:National Resilience, Inc. based inSan Diego, CALIFORNIA (USA)
Innovators in immunotherapy, stem cell therapy, and regenerative medicine will find like-minded champions at ...
Manufactured by:Vetherapy based inSan Francisco, CALIFORNIA (USA)
Our proprietary stem cell therapies are optimized for inflammatory, auto-immune and neurologic disorders in pets and can be delivered ready to inject anywhere in the world. We provide stem cells for cats, dogs and ...
Manufactured by:Kangstem Biotech Co., Ltd. based inGangnam-gu, SOUTH KOREA
FURESTEM-RA Inj. targets moderate to severe rheumatoid arthritis ...
Manufactured by:Magellan based inBox Hill North, AUSTRALIA
Our product MAG200 has successfully completed Phase I/II of a clinical trials on the use of allogeneic (donor) off-the-shelf stem cell therapy for OA. The pivotal Phase III trial is planned to commence 2023. ...
Manufactured by:ASC Therapeutics based inMilpitas, CALIFORNIA (USA)
Stem cells, including inducible pluripotent stem cells, mesenchymal stem cells (MSCs) and other primary pluripotent cells, hold the potential to treat a variety of diseases. We are currently focusing on developing an allogeneic decidua stromal cells (DSCs)-based platform to treat severe immune-mediated diseases. Our pre-clinical and clinical data suggest that the immunomodulatory activity of DSCs ...
Manufactured by:T-Cure BioScience, Inc. based inSherman Oaks, CALIFORNIA (USA)
The HERV-E target is one of the quiescent Human Endogenous Retro-Virus (HERV) sequences that are turned on during tumor development. Its expression in renal cell carcinoma (RCC) is highly selective, with no transcripts detected in any normal tissues. It is expressed in 85% of clear cell RCC (ccRCC). The TCR molecule was identified in a responding patient, following allogeneic stem cell therapy. ...
Manufactured by:Genenta Science based inMilano, ITALY
Temferon™ is a lenti-virus based hematopoietic stem progenitor cell immuno-gene therapy enabling controlled and targeted interferon-α expression within cancers. Biologically delivered via engineered tumor infiltrating monocytes (Tie2 expressing monocytes – TEMs), Temferon™ generates immune responses to any TEMs positive tumors. As such the biological readouts and safety of ...
Manufactured by:Stemedica Cell Technologies, Inc. based inSan Diego, CALIFORNIA (USA)
There are two types of cells currently used for investigating progenitor cell therapies for degenerative diseases: autologous and allogeneic. Autologous progenitor cells are derived from the patient being treated. They are retrieved surgically, expanded, and transplanted back into the same patient. Allogeneic progenitor cells are derived from healthy volunteers or organ donations. These cells ...
Manufactured by:Kangstem Biotech Co., Ltd. based inGangnam-gu, SOUTH KOREA
FURESTEM-OA Kit Inj., a therapeutic biological product, is composed of allogeneic umbilical cord blood-derived mesenchymal stem cells (FURESTEM-OA Inj.), as a major active component for repairing tissue damage, and cartilage acellular matrix (CAM Inj.), a medical device, as a supportive material. This convergence medical product is under development for the treatment of osteoarthritis patients ...
Manufactured by:The Electrospinning Company Ltd. based inDidcot, UNITED KINGDOM
The goal of this study was to investigate whether a biocomposite electrospun scaffold has the ability to induce differentiation of hMSCs into osteogenic lineage without specific growth factors. This study was conducted by researchers at the University of Malaya. Three scaffolds were generated by The Electrospinning Company for this purpose: PLLA + collagen, PLLA + hydroxyapatite (HA) and PLLA + ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
Morquio B, also known as Mucopolysaccharidosis type IV (MPS IV), is a progressive disease mostly impacting the skeleton, caused by mutations in GLB1, the gene that encodes the beta-galactosidase (GLB) enzyme. These mutations result in the misfolding and subsequent dysfunction of GLB, which leads to the toxic substrate accumulation of keratan sulfate in organs and tissues. Very limited and ...
Manufactured by:Kangstem Biotech Co., Ltd. based inGangnam-gu, SOUTH KOREA
Kangstem Biotech is developing stem cell therapeutic products for patients suffering from rare and incurable diseases using umbilical cord blood-derived stem ...
Manufactured by:MDL Srl based inDelebio, ITALY
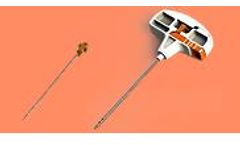
Selective multiradial, micro-aspiration system of autologous bone marrow tissue for stem cell therapy. ...
by:Gain Therapeutics, Inc. based inBethesda, MARYLAND (USA)
GM1 Gangliosidosis is a hereditary, progressive disease mostly impacting neurons in the brain and spinal cord, caused by mutations in GLB1, the gene that encodes the beta-galactosidase (GLB) enzyme. These mutations result in the misfolding and subsequent dysfunction of GLB, which leads to the toxic substrate accumulation of GM1 ganglioside in organs and tissues. Very limited and investigational ...
Manufactured by:KORU Medical Systems based inMahwah, NEW JERSEY (USA)
Primary Immunodeficiency Diseases (PIDD) are a group of over 350 chronic disorders resulting from defects in the body's immune system that are caused by hereditary or genetic defects. PIDD patients have an increased susceptibility to infection due to their defective immune response, enduring recurrent health problems and often developing serious illnesses. With proper medical care, many patients ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
Cell Therapy Systems—a proven choice for clinical cell therapy manufacturing. As you move from basic cell therapy research to the clinic, high-quality GMP-grade cell therapy ancillary materials and proper documentation are essential to getting it right the first time. Cell Therapy Systems (CTS) products provide a proven choice for clinical stem cell therapy and immunotherapy research and ...
by:Aegle Therapeutics based inWoburn, MASSACHUSETTS (USA)
Aegle’s founder Dr. Evangelos Badiavas and his team have spent decades researching clinical applications of mesenchymal stem cells (“MSCs”) in severe dermatological disorders, including conducting clinical trials in burns and wounds under multiple INDs. In these trials, patients experienced accelerated healing, little to no scarring, total wound closure and tissue regeneration. ...
Manufactured by:U.S. Stem Cell, Inc. (USRM) based inSunrise, FLORIDA (USA)
U.S. Stem Cell’s lead product candidate is MyoCell®, a muscle stem cell therapy that is intended to improve cardiac function months or even years after a patient has suffered severe heart damage due to a heart attack. The procedure involves a physician removing a small amount of muscle from the patient’s thigh. From this muscle specimen, muscle stem cells (also called myoblasts) ...
by:VetStem Biopharma based inPoway, CALIFORNIA (USA)
The StemInsure® process can be performed before your dog has arthritis or an injury so that when your dog needs stem cells, no additional fat collection surgery is ...