Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Osteoconductive Equipment Supplied In Europe
37 equipment items found
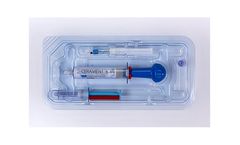
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite is highly osteoconductive, promoting bone ingrowth. The characteristics of CERAMENT BONE VOID FILLER make it ideal for minimally invasive surgery and open procedures ...
Manufactured by:Inion Oy based inTampere, FINLAND
The fully threaded Inion FixOn™ Biocomposite Suture Anchor is made of state-of-the-art biocomposite material containing PLGA and osteoconductive β-TCP. The products are provided sterile and preloaded on a practical, single-use driver with two or three nonabsorbable sutures. Inion FixOn™ Biocomposite Suture Anchors provide excellent performance over the healing ...
Manufactured by:Microport Orthopedics Inc. based inArlington, TENNESSEE (USA)
Alongside the medial-pivot knee system’s clinically demonstrated design, built on the latest kinematic evidence of the natural stability and motion of the knee, BioFoam® cancellous titanium is uniquely suited to meet the challenges of today’s patient population.1 By offering surgeons unparalleled biologic fixation and fixation, this novel material provides a true trabecular ...
Manufactured by:Biovision GmbH based inIlmenau, GERMANY
Potential risks of infection involved in the use or processing of biological material (HIV, BSE, inter alia) are non-existent with BetaBASE. BetaBASE has an interconnected pore system with micropores and macropores that reproduce the well-known osteoconductive effect almost perfectly. Osteoblasts and blood vessels are able to proliferate rapidly in the open pore system and grow ...
Manufactured by:Heraeus Medical GmbH based inWehrheim, GERMANY
Made from resorbable calcium phosphate, heracure 3D forms microcrystalline hydroxyapatite, ensuring biocompatibility and efficient integration as it gradually resorbs and is replaced by endogenous bone. Its interconnected pore system facilitates osteoconduction, tissue ingrowth, and osteointegration, providing structural stability while being compatible with additional autologous ...
Manufactured by:Medosis based inSofia, BULGARIA
This material gradually degrades to harmless byproducts, specifically carbon dioxide and water, facilitating a seamless integration with human physiology. The micro and macro porous architecture is crafted to mimic the structure of natural bone, enhancing osteoconductivity and supporting new bone formation. When applied in dental procedures such as ridge augmentation and sinus ...
Manufactured by:EOS MEDICAL SOLUTIONS (EOSMED) based inSofia, BULGARIA
Biongraft is an injectable and formable paste bone graft based on hydrogel and Beta-tricalcium phosphate (β-TCP), including ZrO2 nanoparticles for antibacterial e?cacy. Biongraft is a safe and fully biocompatible material which is designed to act as an osteoconductive sca?old to support the ingrowth and fusion of adjacent viable bone when placed in an osseous environment ...
Manufactured by:SYMATESE based inCHAPONOST, FRANCE
It is composed of a collagen structure in which ceramised hydroxyapatite granules are dispersed. The granules of hydroxyapatite give the material its osteoconductive properties. The hydroxyapatite is slowly resorbed. As a collagen medical device, COLLAPAT® II its strong haemostatic power and is completely resorbable in a few weeks. The collagen type I is extracted from bovine ...
Manufactured by:Medosis based inSofia, BULGARIA
It is composed of a hydrogel and Beta-tricalcium Phosphate (β-TCP) matrix, enhanced with ZrO2 nanoparticles to provide antibacterial properties. The material serves as an osteoconductive scaffold, facilitating the growth and fusion of surrounding bone tissue in osseous environments. This biocompatible material acts as a bone void filler, designed to be gradually resorbed and ...
Manufactured by:Medosis based inSofia, BULGARIA
For structural integration, Biongraft Granules and Sticks exhibit macroporosity that allows deep bone cell invasion, with particle sizes varying from 0.25 mm to 2 mm to promote mechanical interlocking. These materials are 100% synthetic, osteoconductive, biodegradable, and radiopaque. Indicative of their osteoinductive nature, new bone formation is observed within two months ...
Manufactured by:Neo Medical Sa based inVillette, SWITZERLAND
Neo Cage System Technology. We integrate a fourth dimension with 3D printing: ...
Manufactured by:Biocomposites based inKeele, UNITED KINGDOM
Biosteon is a synthetic calcium composite technology used for interference screws in the reconstruction of anterior cruciate ligaments and suture anchors for rotator cuff ...
Manufactured by:Locate Bio Limited based inBeeston, UNITED KINGDOM
LDGraft uses a powerful protein called rhBMP-2 to drive cell differentiation and bone formation. rhBMP-2 is the most studied growth factor in bone grafting and is the only growth factor which has so far been able to demonstrate equivalent fusion to autograft in lumbar fusion as part of a Tier 1 study. At Locate Bio, we are working hard on a next generation of rhBMP-2 growth factor ...
Manufactured by:Teknimed based inL’union, FRANCE
TRIHA+® is a synthetic monophasic ceramic made of tricalcium phosphate (TCP). Tricalcium phosphate Ca3(PO4)2 provides excellent osteointegration of the ceramic. TRIHA+® is a biocompatible and ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
The Evolution® medial-pivot knee system is designed to answer the limitations of traditional implants by delivering superior flexion stability, anatomic motion, and wear–limiting design ...
Manufactured by:Leader Biomedical Group based inAmsterdam, NETHERLANDS
Cancellous and cortical bone chips with sound structural and mechanical properties for filling of bone loss and/or correcting of ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT V is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic vancomycin ...
Manufactured by:SpineVision based inAntony, FRANCE
Smart’OS is a bone substitute combining in both granules or putty a micro & macroporous structure with the well known association of β-TCP & Hydroxy Apatite to enhance bone fusion. ...
Manufactured by:SpineVision based inAntony, FRANCE
The Hexanium ACIF cage is a 3D printed standalone cervical interbody system made of titanium. It combines titanium rough surface, high porosity and honeycomb structure. The user-friendly one-step locking mechanism was developed to make the surgery ...