Show results for
Refine by
In Vitro Diagnostic Equipment Supplied In Germany
33 equipment items found
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
The TH-11 is the next step towards a fully automated urinalysis work area. Opening all common urine tubes on the market ? no matter whether push top or screw top – the TH-11 eliminates another manual step in your workflow. With its direct connection to the UN-Series analysers – either UC-3500 or UF-5000/UF-4000 – it seamlessly transports the decapped urine sample tubes to these ...
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
The new UC-1000 integrates within your urinalysis workflow in many ways. Whether you are looking for a backup for your UC-3500 fully automated system or require a smaller system for processing individual test strip order requests, UC-1000 is equally well suited. You can set it up stand-alone or integrated into your UN-Series solution. You can choose between different test strips for the UC-1000: ...
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
The new UC-3500 is a fully automated urine chemistry analyser that offers particularly high clinical value. Urine sample material is dropped onto each pad of a dedicated test strip within the analyser. The entire sequence, starting from sample aspiration, to colour comparison and final output of the results is fully automatic. In addition to the five system parameters, the 11 test strip ...
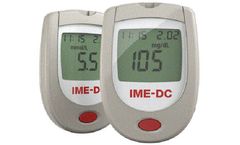
Manufactured by:IME-DC Group based inHof, GERMANY
The iDia blood glucose monitoring system is an in vitro diagnostic tool that is suitable for self-testing. It enables people with diabetes and specialist medical staff to determine blood glucose ...
Manufactured by:IME-DC Group based inHof, GERMANY
The IME-DC blood glucose monitoring system is an in vitro diagnostic tool that is suitable for self-testing. It enables people with diabetes and specialist medical staff to determine blood glucose ...
Manufactured by:DRG Instruments GmbH based inMarburg, GERMANY
The DRG Anti Ovarian Ab ELISA is an enzyme immunoassay for the quantitative in vitro diagnostic measurement of antibodies directed against oocytes in human ...
Manufactured by:in.vent Diagnostica GmbH based inBerlin, GERMANY
Human tissue serves as a starting material for the extraction of various proteins. Freshly frozen tissue is essential for biomarker research and the successful development of in-vitro diagnostics. in.vent provides standardised and validated fabrics of the highest quality according to individual requests. Thyroid tissues are used, for example, for the extraction ...
Manufactured by:Clinical Diagnostics, a division of R-Biopharm AG based inDarmstadt, GERMANY
For in vitro diagnostic use. The RIDA®UNITY Universal Extraction Kit is intended for the automated isolation and purification of nucleic acids from defined human biological samples and is carried out on the RIDA®UNITY System. The product is intended for professional ...
Manufactured by:DRG Instruments GmbH based inMarburg, GERMANY
The DRG Cortisol CLIA is a chemiluminescence immunoassay for the quantitative in vitro diagnostic measurement of Cortisol in serum and plasma (EDTA, ...
Manufactured by:DRG Instruments GmbH based inMarburg, GERMANY
The DRG Estradiol CLIA is a chemiluminescence immunoassay for the quantitative in vitro diagnostic measurement of Estradiol in serum and ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
Rheumatoid Factor IgM is an indirect solid phase enzyme immunoassay ( ELISA ) for the quantitative measurement of IgM class rheumatoid factor antibodies in human serum or plasma. The assay is intended for in vitro diagnostic use only as an aid in the determination of rheumatoid arthritis ( RA ). ...
Manufactured by:DRG Instruments GmbH based inMarburg, GERMANY
The DRG Aldosterone ELISA is a manual enzyme immunoassay for the quantitative measurement of aldosterone in human serum or plasma (EDTA, Li-heparin or citrate plasma) or urine. For in vitro diagnostic use. For laboratory professional use. The device is intended to be used as an aid to diagnosis of primary and secondary aldosteronism. The device is not intended ...
Manufactured by:altona Diagnostics GmbH based inHamburg, GERMANY
The AltoStar Purification Kit 1.5 uses magnetic particle technology and is intended to be used for automated isolation and purification of nucleic acids from specified human specimens for in vitro diagnostic purposes. It permits simultaneous purification of RNA and DNA from multiple sample ...
Manufactured by:Clinical Diagnostics, a division of R-Biopharm AG based inDarmstadt, GERMANY
For in vitro diagnostic use. The RIDA®UNITY Internal Control Kit is intended for control of automated isolation and purification, amplification, and detection of nucleic acids in connection with the RIDA®UNITY Universal Extraction Kit and the RIDA®UNITY PCR kits on the RIDA®UNITY System. ...
Manufactured by:BIORON Diagnostics GmbH based inRömerberg, GERMANY
RealLine Chlamydia trachomatis Kits detect Chlamydia trachomatis plasmid and genomic DNA in clinical samples by Real-Time PCR. The kits are designed for in vitro diagnostics and provide qualitative ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
New "ray of light" in Vitamin D testing. Lumipulse G 25-OH Vitamin D is an immunoreaction cartridge for in vitro diagnostic (IVD) use with the LUMIPULSE G System for the quantitative determination of 25-hydroxyvitamin D (25-OH-D) in human serum or plasma to be used in the assessment of Vitamin D ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
Rheumatoid Factor IgG is an indirect solid phase enzyme immunoassay ( ELISA ) for the quantitative measurement of IgG class rheumatoid factor antibodies in human serum or plasma ( Citrate plasma, Na-heparin Plasma, EDTA plasma ). The assay is intended for in vitro diagnostic use only as an aid in the diagnosis of rheumatoid arthritis ( RA ). ...
Manufactured by:DRG Instruments GmbH based inMarburg, GERMANY
The DRG C-Peptide ELISA is an enzyme immunoassay for the quantitative in vitro diagnostic measurement of C-Peptide in serum, plasma (EDTA, lithium heparin or citrate plasma) and ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
CanChek Tumor Marker Control Serum is intended for use as an assayed quality control serum to monitor the accuracy of laboratory testing for neuron specific enolase (NSE), S100B protein and squamous cell carcinoma antigen (SCCA). The use of a quality control serum is indicated to verify the performance characteristics of an in vitro diagnostic laboratory test and ...
Manufactured by:InVivo BioTech Services GmbH a BRUKER Company based inHennigsdorf, GERMANY
Your challenging clone is in good hands with us supported by special equipment and many years of know-how. Our facilities allow the production of up to 1 kg monoclonal antibodies for use in research, human in-vitro diagnostic, preclinical development, veterinary diagnostics, food and feed safety. Proprietary serum-free media and optimized ...