- Home
- Equipment
- north america
- heart valve
Show results for
Refine by
Heart Valve Equipment Supplied In North America
63 equipment items found
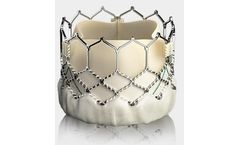
Manufactured by:Anteris Technologies Limited based inToowong, AUSTRALIA
Evidence of Long-Term Durability: 25 patients were followed for up to 10 years; No graft failure, thromboembolic events, infections or device-related reinterventions; Echocardiographic assessment demonstrated the absence of calcification in all ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Bioprosthetic heart valves may have addressed some of the limitations of mechanical valves but still carry their own: gradual degeneration such as pannus growth, leaflet fibrosis and calcification, delamination of the connective tissue, and emergence of ruptures and perforations all eventually lead to valve failure (Kostyunin et ...
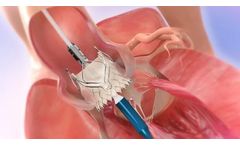
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
The On-X Aortic Valve is a newer generation heart valve made of a unique material and design characteristics compared with earlier generations of mechanical heart valves. The On-X Aortic Valve is the only mechanical valve with FDA and CE approval to be used safely with less blood ...
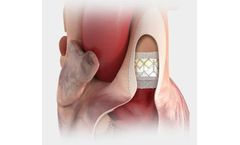
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in ...
Manufactured by:JenaValve Technology, Inc. based inIrvine, CALIFORNIA (USA)
Nitinol Support Frame: Self-Expanding Design – Available in (3) Sizes. Large Cells for Coronary ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Anteris Technologies Limited based inToowong, AUSTRALIA
The World’s First & Only 3D Single-piece Aortic Valve. DurAVR™ heart valve has the potential to deliver a functional cure in the treatment of Severe Aortic Stenosis. The unique design of DurAVR™ coupled with the ADAPT® technology provide the potential to deliver a more durable heart ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
CryoValve SG Pulmonary Human Heart Valves are indicated for the replacement of diseased, damaged, malformed, or malfunctioning native or prosthetic pulmonary valves. They may also be used in the replacement of native pulmonary valves when the Ross Procedure is performed. Pulmonary heart valve ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and ...
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
Abbott's mechanical heart valves offer a reliable solution for patients requiring aortic and mitral valve replacements. Featuring a bileaflet design, these valves prioritize low thrombogenicity to ensure excellent patient outcomes. The pivot guard design provides significant benefits during and after implantation. With specific ...
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
The Epic Plus Mitral and Aortic Stented Tissue Valves from Abbott represent the latest generation of the Epic Platform, designed for patients requiring heart valve replacement due to diseased, damaged, or malfunctioning valves. These bioprosthetic valves are engineered with advanced features aimed at enhancing ...
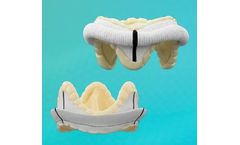
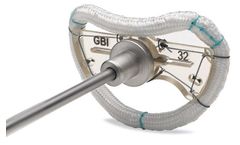
Manufactured by:Genesee BioMedical, Inc. based inDenver, COLORADO (USA)
True Physiological Repair: The TruForm Sievers Annuloplasty Ring is designed to maintain optimum conditions throughout the cardiac cycle, allowing the valve to remain in a mechanically reduced state of stress. Implantation is facilitated as the ring is congruent to surgical landmarks; the ring fits to the surgically relevant structures. The TruForm Sievers Annuloplasty Ring addresses the shape of ...
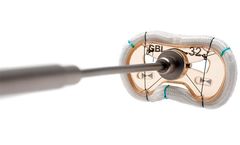
Manufactured by:Genesee BioMedical, Inc. based inDenver, COLORADO (USA)
Geometrically designed to restore leaflet coaptation and reduce mitral regurgitation (caused by the enlargement of the left ...
Manufactured by:Genesee BioMedical, Inc. based inDenver, COLORADO (USA)
Design Advantage: Unique braided material provides easy needle penetration and reduces risk of bunching or crimping. Five markers are provided as landmarks for suture ...