Show results for
Refine by
Colonic Equipment Supplied In Usa Massachusetts
20 equipment items found
Manufactured by:Emulate based inBoston, MASSACHUSETTS (USA)
Investigate mechanisms of colon inflammation and barrier disruption and evaluate the efficacy of anti-inflammatory drug candidates in an organoid-derived ...
Manufactured by:Instrument Technology, Inc. (ITI) based inWestfield, MASSACHUSETTS (USA)
Key Features: Precision optics, ergonomic design. Applications: Colon cancer screening and ...
Manufactured by:Adiso Therapeutics based inConcord, MASSACHUSETTS (USA)
ADS051 is an oral, gut-restricted, small molecule modulator of neutrophil trafficking and activation for the treatment of ulcerative colitis. ADS051 blocks MRP2 release of HXA3 gradient, while also blocking FPR1 activation of neutrophils within the colonic epithelium. Unlike currently available therapies, this addresses neutrophil-mediated tissue damage, a hallmark of UC ...
Manufactured by:Adiso Therapeutics based inConcord, MASSACHUSETTS (USA)
A naturally occurring live biotherapeutic product, ADS024 modulates inflammation as a single strain multimodal LBP and has been shown to reduce neutrophil trafficking through the colonic epithelium. ADS024 is manufactured from a pure, clonal bacterial cell bank, yielding a standardized lyophilized drug product eliminating the need to directly source from donor fecal material. ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE303 is an orally administered, rationally-defined bacterial consortium candidate being developed for high-risk Clostridioides difficile (CDI) infection. VE303 consists of 8 types of clonal human commensal bacteria strains selected for their ability to provide colonization resistance to C. difficile and manufactured under cGMP conditions. It is produced under cGMP conditions ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE707 is a preclinical discovery program for the prevention of infection and colonization recurrence of several multi-drug resistant organisms (MDROs) including carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta lactamase producers (ESBL), and vancomycin-resistant Enterococci (VRE), which are some of the most common hospital-acquired ...
Manufactured by:Gyn Surgical Solutions based inMarlborough, MASSACHUSETTS (USA)
The Panther Fusion MRSA assay† is designed for the qualitative detection and differentiation of Staphylococcus aureus (SA) and methicillin-resistant Staphylococcus aureus (MRSA) DNA from nasal swabs in patients at risk for nasal colonization. It is intended to aid in the prevention and control of SA and MRSA infections in healthcare settings. The Panther Fusion MRSA assay ...
Manufactured by:FCI based inPembroke, MASSACHUSETTS (USA)
It is particularly designed for enucleation, evisceration, and secondary implantation procedures. The implant features a porosity range between 40% and 60%, facilitating the optimal colonization of fibrovascular tissues which is crucial for integration and healing. The anterior surface of the implant is smooth, equipped with eight suture tunnels enabling efficient needle ...
Manufactured by:Anika Therapeutics, Inc. based inBedford, MASSACHUSETTS (USA)
Hyalomatrix® is a sterile, biodegradable, flexible and conformable advanced wound care device, made entirely of HYAFF®, a derivative of hyaluronic acid (HA). The matrix provides a 3-D scaffold for cellular invasion and capillary growth, able to be colonized by fibroblasts and onto which extracellular matrix components are regularly laid down, facilitating an ordered ...
Premium
Distributed by:FIBERSCOPE.net based inWinnipeg, MANITOBA (CANADA)
The FVG Video Gastroscopes also feature a waterproof insertion probe with 4-way tip articulation, an instrument channel for flexible forceps, additional channels for air/water, and suction (additional unit required), a wide angle 140 degree field of view, a focal range of 5mm – 100mm from the tip, and a 200 watt Halogen light source incorporated into the design. For customers looking to ...
by:Iterative Health, Inc. based inCambridge, MASSACHUSETTS (USA)
Accuracy is everything. Better adenoma detection can boost any GI practice’s standard of care. SKOUT uses advanced computer-vision technology to better recognize colorectal polyps. Gastroenterologists get real-time feedback during colonoscopies, making it easier to detect lesions early on. SKOUT augments but does not replace physician ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE202 is an orally administered rationally-defined bacterial consortium candidate being developed for the potential treatment of inflammatory bowel disease (IBD). VE202 consists of clonal human commensal bacteria strains selected for their ability to impact the number and activity of regulatory T cells in the gut mucosa and manufactured under cGMP ...
by:Huabio based inWoburn, MASSACHUSETTS (USA)
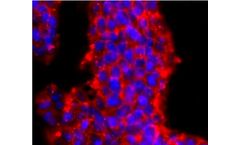
ICC staining of PRMT1 in D3 cells (red). Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA for 15 minutes at room temperature. Cells were probed with the primary antibody (ET1704-19, 1/50) for 1 hour at room temperature, washed with PBS. Alexa Fluor®594 Goat anti-Rabbit IgG was used as the secondary ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A new generation of multifunctional oncolytic virus, TG6002 has been designed to combine the mechanism of oncolysis (targeted destruction of the cancer cell) with the production of chemotherapy (5-FU), directly into the ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
From Ruslan Medzhitov's discovery of toll-like receptors (TLRs) as the key immune system receptors that detect infection, which spawned the field of innate immunity, to Dan Littman's efforts to identify and clone CD4 and CD8, molecules on the surface of T cells that are crucial to immune recognition and response, Vedanta's scientific co-founders have pioneered the current understanding of how the ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-301 is an investigational, oral, rationally-designed, fermented microbiome therapeutic for the treatment of mild-to-moderate ulcerative colitis (UC). SER-301 is a consortium of multiple bacterial strains that is manufactured by fermenting each strain individually and then combining to form drug product. ...
by:Huabio based inWoburn, MASSACHUSETTS (USA)
Western blot analysis of GARS on Raji cell lysates. Proteins were transferred to a PVDF membrane and blocked with 5% BSA in PBS for 1 hour at room temperature. The primary antibody (ET1610-67, 1/500) was used in 5% BSA at room temperature for 2 hours. Goat Anti-Rabbit IgG - HRP Secondary Antibody (HA1001) at 1:40,000 dilution was used for 1 hour at room ...
Manufactured by:Seres Therapeutics, Inc. based inCambridge, MASSACHUSETTS (USA)
SER-287 is an investigational, oral, biologically-derived microbiome therapeutic for the treatment of ulcerative colitis (UC). SER-287 is a consortium of multiple bacterial spores manufactured by fractionating and purifying targeted bacteria from stool of healthy human donors. SER-287 has been granted Fast Track designation and Orphan Drug designation by the FDA. A completed SER-287 Phase 1b ...
Manufactured by:Finch Therapeutics Group, Inc. based inSomerville, MASSACHUSETTS (USA)
Our lead product candidate, CP101, is an orally administered, Complete Consortia product candidate initially targeting the prevention of recurrent CDI. The Centers for Disease Control and Prevention considers CDI to be one of the top three most urgent antibiotic resistant threats and the most common cause of healthcare associated infection in the United ...
Manufactured by:SRS Medical based inN. Billerica, MASSACHUSETTS (USA)
The Spanner® Prostatic Stent is a temporary stent inserted into the urethra at the neck of the bladder to maintain urine flow. The Spanner benefits men with bladder outlet obstruction (BOO) by reducing elevated post void residual (PVR), improving voiding symptoms, and allowing for voluntary urination. As an alternative to a Foley catheter, it allows men to naturally fill and empty their ...