Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Approved Drug Equipment Supplied In Usa
57 equipment items found
by:Curemark based inRye Brook, NEW YORK (USA)
Centers for Disease Control and Prevention estimate that 1 in 68 children in the United States have been diagnosed with Autism. There is currently no approved drug on the market that treats the core symptoms of Autism. At Curemark, we are working to change that. Curemark’s lead drug candidate, CM-AT, has received “Fast Track” ...
by:Curemark based inRye Brook, NEW YORK (USA)
Current prescription medications and psychosocial therapies focus on ameliorating primary symptoms. No currently approved drug treats the underlying cause of ADHD, and there is no known cure. Our pipeline includes a Phase III-ready drug product for ADHD. Preliminary studies with patients diagnosed with ADHD and taking prescribed ADHD medication ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
In multiple myeloma, malignant plasma cells accumulate in the bone marrow and produce abnormal antibodies called M proteins, which can cause kidney damage, bone destruction, and impaired immune function. While multiple approved drugs with novel mechanisms have improved disease management over the past decade, multiple myeloma is rarely curable and a significant ...
Manufactured by:Cerevast Medical, Inc. based inBothell, WASHINGTON (USA)
The formation or deposition of these clots in the arterial vessels of the brain is one of the primary causes of ischemic stroke. Intravenous thrombolytic therapy (tPA/alteplase, the only approved drug for the treatment of ischemic stroke) works by converting plasminogen in the blood stream to plasmin, the active enzyme that dissolves ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Mobilized Leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate the migration of CD34+ hematopoietic stem and progenitor cells (HSPCs) from the bone marrow niche into the circulation. Mobilized peripheral blood, enriched in single-donor CD34+ HSPCs is collected through leukapheresis using the Spectra ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
N,N-Dimethylacetamide (DMAc) is an organic solvent with blood-brain transmissibility and an FDA-approved drug excipient. N, N-dimethylacetamide exerts anti-inflammatory activity by inhibiting the NF-κB signaling pathway. N, N-dimethylacetamide can be used in studies of weight gain caused by a high-fat diet and neuroinflammation in Alzheimer's ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
The emerging global threat from COVID-19 demanded immediate action. With cases rising rapidly, we set up a specialist team to use our AI-drug discovery platform to search for existing drugs — already proven safe — that could be repurposed to treat the virus. We are the only AI company to successfully identify a drug to treat COVID-19. ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Dalbavancin (INN, trade name Dalvance) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin.The Food and Drug Administration approved dalbavancin for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible ...
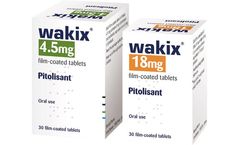
Manufactured by:RareStone Group based inShanghai, CHINA
Pitolisant is a selective histamine H3-receptor antagonist/inverse agonist, which increases the release of the brain chemical histamine to increase a patient's wakefulness and alertness. The drug was approved by the European Medicines Agency (EMA) in 2016 for the treatment of narcolepsy in adults with or with0out cataplexy and is marketed in major countries in ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
In October 2018, SIGA entered into a Cooperative Research and Development Agreement (CRADA) with the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) with a focus on Good Laboratory Practice (GLP) studies of TPOXX® for PEP using a U.S. Food and Drug Administration (FDA)-approved non-human primate model of TPOXX® efficacy ...
Manufactured by:Eleven P15 based inDenver, COLORADO (USA)
Idiopathic Pulmonary Fibrosis (IPF) is a chronic, fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and localized to the lung. IPF is a fatal disease with median survival at 3-5 years. Two drugs have been approved for treatment of IPF, but these drugs fail to reverse scarred lung tissue or to ...
by:Starton Therapeutics based inParamus, NEW JERSEY (USA)
STAR-LLD is in development in two continuous delivery systems: subcutaneous and transdermal. STAR-LLD is being developed to establish the only IMiD (immunomodulatory drug) approved for CLL. CLL is the most common form of leukemia. Lenalidomide has been shown to be efficacious and well tolerated in CLL previous randomized controlled trials did not obtain an ...
Manufactured by:Amivas (US), LLC based inFrederick, MARYLAND (USA)
Artesunate for Injection is an antimalarial indicated for the initial treatment of severe malaria in adults and ...
Manufactured by:EndoLogic LLC based inClifton, NEW JERSEY (USA)
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety ...
Manufactured by:BioQ Pharma based inSan Francisco, CALIFORNIA (USA)
invenious connect-and-go technology is already being used to deliver infusibles in a patented range of infusion systems. Our products are thoughtfully-designed to each drug – with bespoke solutions for: Drugs requiring complex weight-based dosing. Cytotoxic drugs. Drugs that need to be reconstituted or diluted at time of use. Drug-drug combinations, including for cold-chain ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Oral TPOXX® (tecovirimat) is the first drug approved by the U.S. Food and Drug Administration (FDA) that is specifically indicated for the treatment of smallpox disease in adults and pediatric patients weighing at least 13 kg. (Prescription Label) TPOXX® inhibits viral maturation of variola virus (the virus that causes smallpox) and other ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:Quanterix Corporation based inBillerica, MASSACHUSETTS (USA)
For the first time, oncology and immuno-oncology researchers and others who rely on multiplexing capabilities have an easy-to-use platform to help optimize workflows, speed up their research and ultimately accelerate drug ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UroGen is developing UGN-102 (mitomycin) for intravesical solution as a primary nonsurgical alternative to repetitive TURBT. UGN-102 is an investigational formulation that utilizes our innovative technology, RTGel™ reverse-thermal hydrogel, for the treatment of low-grade NMIBC. Instilled via standard catheters, UGN-102 is designed to dwell for a period of several hours before being excreted ...