Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Disease Monitoring Equipment Supplied In Usa
28 equipment items found
Manufactured by:Creatv MicroTech, Inc. based inRockville, MARYLAND (USA)
Personalized Cancer Therapy and Disease Monitoring: “Liquid biopsy” using circulating tumor cells (CTCs) is potentially a minimally invasive alternative to traditional tissue biopsy to determine cancer therapy. Enumeration of CTCs may be used to indicate prognosis and to monitor treatment. ...
Manufactured by:Blue Earth Diagnostics based inOxford, UNITED KINGDOM
Nuclear medicine is a medical speciality that uses targeted radioactive compounds, called radiopharmaceuticals, to diagnose, stage, treat and monitor diseases. Nuclear medicine can be divided into two categories: diagnostic nuclear medicine (or molecular imaging) and therapeutic nuclear medicine (or theranostics). The nuclear medicine market is predicted to reach ...
Manufactured by:AccuGenomics, Inc. based inWilmington, NORTH CAROLINA (USA)
Accukit™ ONCO1LB contains a blend of 13 synthetic DNA internal standards (IS) to clinically actionable oncogenic regions of the genome (Table 1). The mixtures are physically and enzymatically fragmented to an average size of ~165bp and are suitable for use as routine spike-in controls for cell-free DNA assays. Accukit Onco1LB internal standards are spiked into each specimen prior to library ...
Manufactured by:UMA Instruments based inDayton, VIRGINIA (USA)
The PA-100 is an enhanced Collens Sphygmo-Oscillometer developed to assist physicians in diagnosing peripheral vascular disease and to monitor arterial grafts. UMA Plethysmo Analyzer accurately measures volume changes of blood flow in ...
by:Precision Monitoring based inBurleson, TEXAS (USA)
The cardiac device is intended for use by patients who either have, or are at risk of having cardiac disease and those that demonstrate intermittent symptoms indicated by cardiac disease and require cardiac monitoring on a continuing basis. The device continuously records ECG data and, upon detection by an ECG analysis algorithm or manually ...
Manufactured by:Guardant Health, Inc. based inRedwood City, CALIFORNIA (USA)
Our Guardant Reveal™ test is the first blood-only test that detects residual and recurrent disease in two weeks, without the need for a tissue biopsy. The test detects circulating tumor DNA (ctDNA) in blood after surgery to identify patients with residual disease who may benefit most from adjuvant therapy. Our first indication is early-stage colorectal cancer with additional cancer types to ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
Advanced flash capabilities: ColorDome™ has a trillion-to-1 luminance range; Flash duration from nanoseconds to hours; Any color background from RGB and amber LEDs; Easy to use, ultimate performance: Self-calibrating; Full electronic color control; Flash or flicker stimuli in any color & duration; Desktop, cart and floorstand ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
Ideal for Pediatric and Operating Room testing. Features: Advanced flash capabilities, ColorBurst™ has a >10 billion-to-1 luminance range, Flash durations of nanoseconds to minutes, Any color background from red, green & blue LEDs, Easy to use, ultimate performance: Auto calibration, Buttons on handle control testing, User programmable arbitrary waveforms with 1 ms, resolution ...
Manufactured by:LGC SeraCare based inMilford, MASSACHUSETTS (USA)
The Seraseq Tumor Mutation DNA Mix v2 (AF7) HC is a multiplexed mixture of actionable biosynthetic DNA targets precisely blended with a single, well-characterized genomic background. This product is produced under rigorous design control and manufacturing practices to an allelic frequency of 7%, and is offered in a high concentration (HC) format at 25 ng/µL. Designed to assess the overall ...
Manufactured by:MacuLogix based inMiddletown, PENNSYLVANIA (USA)
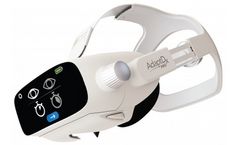
Backed by 20 years of proven clinical research, the AdaptDx Pro® is a revolutionary new way to test patients. Advanced, precision eye tracking technology automatically aligns with the eye to capture an accurate measurement of dark adaptation ...
Manufactured by:Eko Devices, Inc. based inOakland, CALIFORNIA (USA)
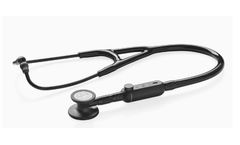
Transform the stethoscope you already own and enhance auscultation through amplification, active noise cancellation, wireless listening, and Eko ...
Manufactured by:BUHLMANN Diagnostics Corp based inAmherst, NEW HAMPSHIRE (USA)
Elevated levels of serum ACE have been measured in patients suffering from various disorders. They often indicate a poor prognosis or rapid progression of the disease. Elevated serum ACE levels are reported in granulomatous-inflammatory diseases such as Sarcoidosis and Mixed-Connective-Tissue Disease (MTCD), Nephropathies associated with Diabetes ...
Manufactured by:Abaxis is now a part of Zoetis based inUnion City, CALIFORNIA (USA)
The VETSCAN VSpro is an easy-to-use coagulation analyzer enabling canine and feline PT (prothrombin time) and aPTT (activated partial thromboplastin time) testing and equine fibrinogen testing. With an incredibly simple and intuitive user interface, it enables you to enhance your clinic’s capabilities and obtain important information on-site within minutes, especially valuable for ...
by:Natera, Inc. based inAustin, TEXAS (USA)
The detailed sequencing report includes FDA-approved treatments, novel treatment approaches and ongoing clinical trials targeting found mutations. Unlike any other tumor profiling assay, Altera also enables molecular residual disease (MRD) monitoring with the Signatera assay. ...
Manufactured by:Athelas Incorporated based inMountain View, CALIFORNIA (USA)
The Athelas Weight Scale allows patients to automatically track their weight from the comfort of their own home. The Athelas Remote Patient Monitoring Weight Scale is a remote-connected device that is ready to use directly out of the box. The weight scale is used by hundreds of patients across the ...
Manufactured by:Eko Devices, Inc. based inOakland, CALIFORNIA (USA)
The 3M Littmann CORE Digital Stethoscope features the outstanding acoustics, comfort and quality of a Littmann cardiology-grade stethoscope enhanced by powerful digital ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
The AmeriDx® Calprotectin (CPTN) Rapid Test Kit is a qualitative test that detects CPTN antigen in human fecal specimens and other body ...
Manufactured by:Notal Vision, Inc. based inManassas, VIRGINIA (USA)
Notal Vision is developing a first-of-its-kind Artificial Intelligence-enabled digital diagnostic for patients with neovascular retina diseases using our patient-operated Home Optical Coherence Tomography device. The first disease in the Notal Home OCT Program pipeline is neovascular Age-related Macular Degeneration ...
Manufactured by:ProUroCare Medical Inc. based inEden Prairie, MINNESOTA (USA)
Our second product, the ProUroVision system, is a proprietary vision technology for thermal therapy systems designed to aid in the treatment of BPH and other prostate diseases. Thermal therapy is a minimally invasive catheter-based therapy that uses microwave technology to precisely and preferentially heat diseased areas of the prostate to a temperature sufficient to cause cell death and is the ...
Manufactured by:Markes International Ltd - a company of the Schauenburg International Group based inBridgend, UNITED KINGDOM
Markes’ BioVOC-2™ breath sampler is a lightweight and simple-to-use device designed for non-invasive biological exposure monitoring of VOCs. Using a design pioneered by the UK Health & Safety Executive, BioVOC-2 collects the last 129 mL of expired breath for transfer to a sorbent tube, and subsequent analysis by thermal desorption. BioVOC-2 is for research use only. Not for use in ...