Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Implant Specific Equipment Supplied In Usa
23 equipment items found
Manufactured by:Exelint International, Co. based inRedondo Beach, CALIFORNIA (USA)
Exel Huber Needles Non-Coring (Right Angle) are also available in a straight angle. The needle is made from SUS – 304 stainless steel construction. The angled tip aids with administering injection in implantable ports. ...
Manufactured by:Voyage Medical Solutions based inLongview, TEXAS (USA)
Our VMS team has created partnerships with multiple surgical device companies for our surgeons’ needs. Contact us today for a complete portfolio. If you don’t see a specific implant product you’re looking for, feel free to contact us to see if we can get you the durable medical equipment you ...
by:Nelson Laboratories, LLC based inSalt Lake City, UTAH (USA)
Cytotoxicity: MEM elution, 48 hr inc., L929, 24 hr ext ...
by:Nelson Laboratories, LLC based inSalt Lake City, UTAH (USA)
Cytotoxicity: MEM elution, 48 hr inc., L929, 72 hr ext ...
Manufactured by:Conformis based inBillerica, MASSACHUSETTS (USA)
Engineered for low contact stress throughout the range of motion, Conformis knee replacement systems are designed to reduce the risk of polyethylene wear. We use the latest material technology for stronger and more durable polyethylene inserts, and offer two types of polyethylene material: iPoly, and iPoly ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
As part of the AMIS EXPERIENCE offer, the AMIS BIKINI represents an ADVANCED level of our anterior minimally invasive surgical offer that surgeons can experience within the comprehensive AMIS educational program. ...
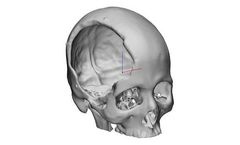
Manufactured by:Techfit Digital Surgery based inDaytona Beach, FLORIDA (USA)
The recommended solution for the correction of large, medium and small defects on patient geometry. Implants are made with the following manufacturing materials: Polyetheretherketone (PEEK) or Titanium (Printed, machined and pre-molded). Implants designed and manufactured according to the anatomy of the patient. ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
AMIS (Anterior Minimally Invasive Surgery) is the only hip replacement technique that follows both an inter muscular and inter nervous path to greatly decrease damage to periarticular structures while preserving tissue. AMIS is a true muscle-sparing procedure supported by the industry’s most detailed surgeon training protocol. ...
by:Harvard Apparatus Regenerative Technology, Inc. formely known as Biostage, Inc. based inHolliston, MASSACHUSETTS (USA)
Following extensive research and development, Biostage has evolved its technology to optimize the interaction of mesenchymal stem cells seeded on a biocompatible scaffold. Preclinical studies suggest that, once implanted, the scaffold seeded with the patient’s own cells signals the stem cell niche to guide the regeneration of a biological ...
Manufactured by:Merete Technologies, Inc. (MTI) based inOakbrook Terrace, ILLINOIS (USA)
Cannulated legs allow accurate placement using K-wires. Low fluoroscopy times are another advantage. The PediatrOS™ RigidTack™ is the only growth arrest implant on the market specifically developed and approved for correcting leg length differences in children and ...
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
Featuring a bileaflet design, these valves prioritize low thrombogenicity to ensure excellent patient outcomes. The pivot guard design provides significant benefits during and after implantation. With specific models like the Regent™ and Masters Series, these valves accommodate a broad range of patient needs, including pediatric cases with the world's ...
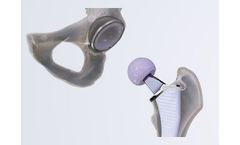
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
The fully porous, distal fixation philosophy of the Arcos One-piece Femoral Revision System offers three cylindrical, forged titanium stem options with a unique size range to address a wide range of femora, including patients of small ...
Manufactured by:SpineAlign Medical, Inc. based inSan Jose, CALIFORNIA (USA)
SpineAlign’s nitinol implant is a dynamic tool with unique advantages for treating painful osteoporotic vertebral compression fractures. SpineAlign offers spine specialists the ability to choose fracture-specific implants in sizes and configurations to fit each patient. The VerteLift Implant’s superior flexibility ...
Manufactured by:Matrix Surgical USA based inNewnan, GEORGIA (US) (USA)
Utilizing the latest and most innovative computer imaging and modeling technologies, Matrix Surgical USA can design precisely fitted patient-specific implants ...
Manufactured by:Conformis based inBillerica, MASSACHUSETTS (USA)
Cordera Match Hip System combines the proven Cordera hip stem with Conformis’ state-of-the-art, personalized pre-operative plans and patient-specific ...
Manufactured by:Techfit Digital Surgery based inDaytona Beach, FLORIDA (USA)
reconstruction of maxillofacial defects, including: Trauma, Genetic diseases, Oncological resections, defects of the Temporomandibular Joint (TMJ), Arthritic Diseases, Anchyloses, Condylar Reabsorb, Avascular Necrosis, Failed Procedures, etc. Solutions include virtual surgical planning, surgical guides and patient-specific implants/plates looking for a more ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Utilizing a proprietary multi-phase calcium phosphate (MCD) abrasive, it delivers precise surface texturing without compromising the structural integrity of the implant. Unlike conventional abrasive blasting methods, which may leave residual contaminants, MATRIX MCD® ensures a residue-free finish, complying with ASTM F86 passivation standards. This technology facilitates ...
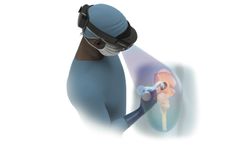
by:HipXpert based inBoston, MASSACHUSETTS (USA)
The HipInsight system represents a significant advancement in the field of orthopedic surgery, specifically for total hip arthroplasty. As the first mixed reality-based digital surgery platform cleared by the FDA for this purpose, the system provides surgeons with a unique capability often likened to 'x-ray vision.' This innovative technology allows medical professionals to ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
Designed specifically for the small but challenging group of patients with isolated, end-stage patello-femoral disease, the KineMatch PFR (Patello-Femoral Replacement) offers a uniquely effective and conservative resurfacing solution. Because the device is precisely pre-fitted to the patient’s anatomy using CT (Computed Tomography) data, no resection of femoral bone is ...
by:SI-BONE, Inc. based inSanta Clara, CALIFORNIA (USA)
Minimally invasive SI joint surgery is the current medical standard of care for SI joint fusion to relieve sacroiliac joint pain. The iFuse Implant System®, available since 2009, is a minimally invasive surgical (MIS) option designed to provide immediate sacroiliac (SI) joint stabilization and allow long-term ...